CLEANING OF DAIRY EQUIPMENT

Aspects of cleaning
The arrangements for cleaning equipment that comes in contact with products are an essential part of a food processing plant. It must be kept in mind that food manufacturers are always obliged to maintain high hygienic standards; this applies both to the equipment and, naturally, to the staff involved in production. This obligation can be considered under three headings:
- 1. Trade obligation
- 2. Moral obligation
- 3. Legal obligation
Trade obligations
Good, wholesome, clean products that keep well and are free from health hazards are obviously good for trade, and customers will buy those same products again. However, if a product is contaminated, does not keep well or is the subject of complaints to the authorities, the reverse is true, and the resulting publicity can be very damaging.
The potential effects of poor cleaning, poor standards and poor quality must be kept in mind at all times.
Moral obligation
Most consumers of dairy products never see the factory or how the products are handled. They trust the company, rely on its reputation, and take it for granted that operations are carried out under the cleanest of conditions by well-trained staff who are continually aware and conscious of these factors.
Legal obligation
The law attempts to protect the customer and purchaser in respect of health and quality. Failure to meet legal obligations, national or local, can result in very severe action, and prosecution proceedings can be very costly.
Prevention is better than cure, and companies are obliged to meet legal requirements and maintain high standards. Milk and milk products by their nature are ideal media for the growth of microorganisms, including many pathogens. As a result of this, there is more legislation concerning milk – its production, handling, processing, packaging, storage and distribution – than any other food product. Each country has its own national, and often local, legislation standards.
Cleaning objectives
Talking about cleaning results, the following terms are used to define the degree of cleanliness:
- Physical cleanliness – removal of all visible dirt from the surface
- Chemical cleanliness – removal, not only of all visible dirt but also of microscopic residues that can be detected by taste or smell but are not visible to the naked eye
- Bacteriological cleanliness – attained by disinfection
- Sterile cleanliness – destruction of all microorganisms
It is important to note that equipment can be bacteriologically clean without necessarily being physically or chemically clean. However, it is easier to achieve bacteriological cleanliness as a matter of routine if the surfaces in question are first rendered at least physically clean.
In dairy cleaning operations, the objective is nearly always to achieve both chemical and bacteriological cleanliness. The equipment surfaces are therefore first thoroughly cleaned with chemical detergents and then disinfected.
Dirt
It’s important to know what kind of dirt is present on the surfaces of the equipment that needs to be cleaned. In the case of a dairy processing line, the dirt will be composed of milk components, which then serve as a matrix and nutrition for bacteria to grow on. Depending on the equipment, processing conditions will impact the state of the milk components and what forces are needed to remove them.
Heated surfaces
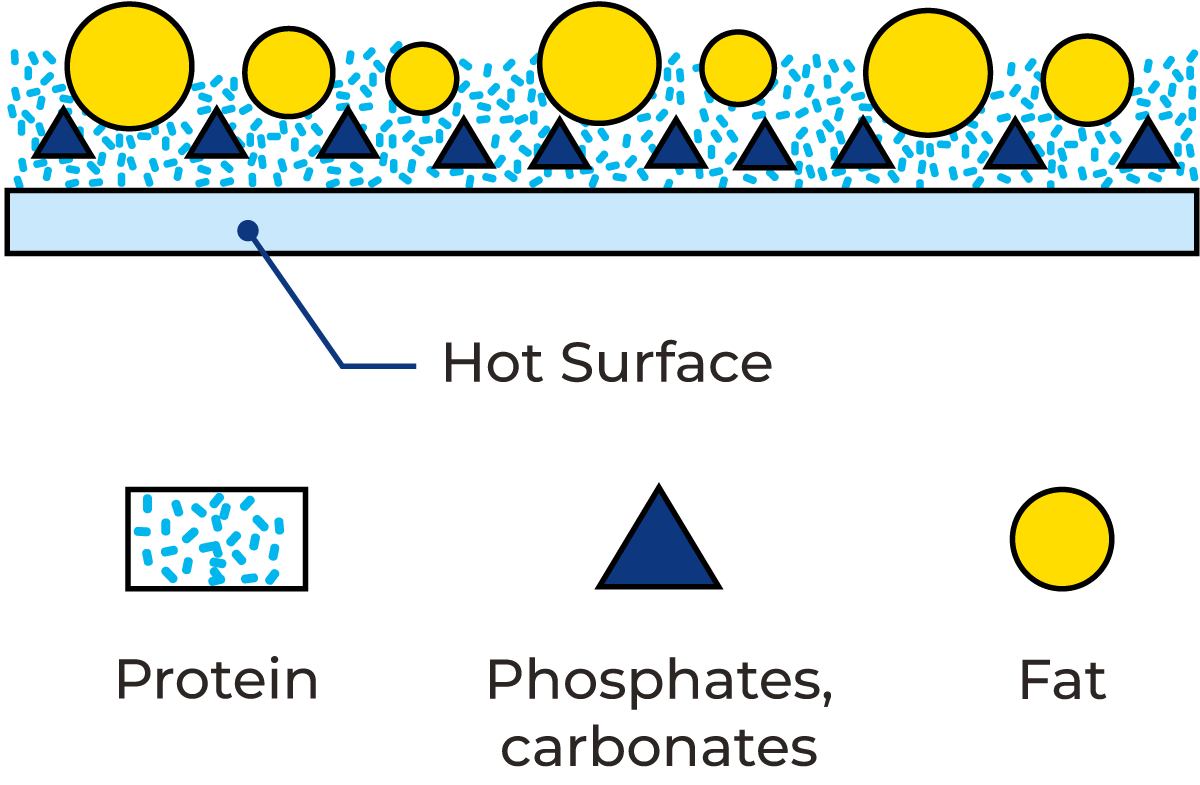
When milk is heated above 60 °C, milk fouling starts to form. This is a deposit consisting of calcium (and magnesium) phosphates, proteins and fat, among other milk components. You can easily see the result of milk fouling forming on heat exchanger plates after a long production run, especially in the heating section and the first part of the regenerative section to follow. The deposits stick tightly to the surfaces, and after runs of more than eight hours, a change of colour from whitish to brownish can also be observed. Figure 22.1 shows a visualisation of milk components forming a fouling layer on a heated surface.
Cold surfaces
A film of milk adheres to the walls of pipelines, pumps, tanks, etc. ("cold" surfaces). When a system is emptied, cleaning should start as soon as possible, otherwise this film will dry out and be harder to remove.
Cleaning procedures
Cleaning of dairy equipment was formerly done (and still is in some places) by people armed with brushes and detergent solutions who had to dismantle equipment and enter tanks to access the surfaces. This was not only laborious but also ineffective; products were often reinfected from imperfectly cleaned equipment.
Circulatory cleaning-in-place (CIP) systems adapted to the various parts of a processing plant have been developed to achieve good cleaning and sanitation results.
Cleaning operations must be performed strictly according to a carefully worked-out procedure in order to attain the required degree of cleanliness. This means that the sequence must be exactly the same every time.
The cleaning cycle in a dairy comprises the following stages:
- Recovery of product residues by scraping, drainage and expulsion with water or compressed air
- Pre-rinsing with water to remove loose dirt
- Cleaning with detergent
- Rinsing with clean water
Each stage requires a certain length of time to achieve an acceptable result. In Table 22.1, some chemical effects and soil characteristics are listed.
Recovery of product residues
All product residues should be recovered from the production line at the end of the run. This is important for three reasons:
- Minimise product losses
- Facilitate cleaning
- Reduce the load on the sewage system, which often means a considerable saving in sewage treatment costs
Time must be allowed for the product to drain from tank walls and pipes. Surfaces coated with solid residues, e.g. in butter-printing machines, must be scraped clean. Before cleaning starts, the remaining milk is forced out of the production lines with water. Wherever possible, the milk in the piping systems is blown or flushed with water to collecting tanks.
Pre-rinsing with water
Pre-rinsing should always be carried out immediately after the production run. Otherwise, the milk residues will dry and stick to the surfaces, making them harder to clean. Milk fat residues are more easily flushed out if the pre-rinsing water is warm, but to avoid coagulation of proteins, the temperature should not exceed 55 °C.
Pre-rinsing must continue until the water leaving the system is clear, as any loose dirt left will increase detergent consumption. If there are dried milk residues on the surfaces, it may be advantageous to soak the equipment. Soaking softens the dirt and makes cleaning more efficient.
The mixture of water and milk from the initial pre-rinsing can be collected in a tank for special processing.
Cleaning with detergent
The dirt on heated surfaces is normally washed off with alkaline and acid detergents, in that order or the reverse order, with intermediate water flushing, whereas cold surfaces are normally cleaned with alkalis and only occasionally with an acid solution.
The most common type of alkali used for cleaning dairy processing equipment is sodium hydroxide, NaOH, and the most common acid is nitric acid, HNO3. However, other types of alkali and acid are also used when preferred by the producer. While most residues can be cleaned using pure alkali and/ or acid solutions, often formulated detergents are used where other ingredients have been added to improve their cleaning efficiency.
Some types of additives that are used in formulated detergents include:
- Surfactants (wetting agents) lower the surface tension, enabling the detergent to wet the surface of the equipment and the dirt more effectively
- Sequestering agents (chelating agents, e.g. EDTA or phosphates) help keep metal ions soluble and prevent them from flocculating
- Builders (e.g. phosphates) prevent soil redeposition by keeping particles suspended in solution
- Oxidation agents (e.g. hydrogen peroxide) boost cleaning through the oxidation of soil on the equipment, creating new sites for detergents to act
- Enzymes (e.g. proteases, amylases, lipases) break down specific types of soil
- Antifoaming agents reduce foam during the cleaning process
Other additives may also be included, and formulated detergent producers are constantly developing new recipes to further improve cleaning efficiency. Formulated detergents can decrease the quantity of chemicals or the time needed for cleaning. However, it is important to understand the impact of added chemicals on the equipment (e.g. chlorine can cause corrosion) and the environment when released in the wastewater.
Several variables must be carefully controlled to ensure satisfactory results with a given detergent solution:
- Concentration of the detergent solution
- Temperature of the detergent solution
- Mechanical effect on the cleaned surfaces (velocity)
- Duration of cleaning (time)
Detergent concentration
The amount of detergent in the solution must be adjusted to the correct concentration before cleaning starts. During cleaning, the solution is diluted with rinsing water and milk residues. Some neutralisation also takes place. It is therefore necessary to check the concentration during cleaning. Failure to do this can seriously affect the result. Checking can be done either manually or automatically. The dosage must always be according to the detergent supplier’s instructions, as increasing the concentration does not necessarily improve the cleaning effect – too high a concentration of detergent may lead to foaming, or in some cases cause protein fouling to become more compact and harder to remove. Furthermore, using too much detergent makes cleaning needlessly expensive, and leads to an increased chemical load on sewage water. NaOH is typically dosed at 0.5 – 2.0 wt% concentration, and nitric acid is typically 0.5 – 1.5 wt%. The exact percentage needed depends on the fouling type and severity, as well as the other chosen parameters for cleaning (time, temperature, flow rate).
Detergent temperature
Generally speaking, the effectiveness of a detergent solution increases with increasing temperature. A blended detergent always has an optimum temperature that should be used.
As a rule of thumb, cleaning with alkaline detergent should be done at the same temperature as the product has been exposed to, but at least 70 °C.
Temperatures of 60 – 75 °C are recommended for cleaning with acid detergents.
Mechanical cleaning effect
In manual cleaning, scrubbing brushes are used to produce the required mechanical scouring effect, (Figure 22.2).
In mechanised cleaning of pipe systems, tanks and other process equipment, the mechanical effect is supplied by the flow velocity. The detergent feed pumps are dimensioned for higher capacities than the product pumps, with flow velocities of 1.5 – 3.0 m/s in the pipes. At these velocities, the liquid flow is very turbulent. This results in a very good scouring effect on the surfaces of the equipment.
Duration of cleaning
The duration of the detergent cleaning phase must be carefully calculated to obtain the optimum cleaning effect. At the same time, the costs of electricity, heating, water and labour must be taken into consideration. It is not sufficient to flush a pipe system with a detergent solution. The detergent must circulate long enough to dissolve the dirt. The time this takes depends on the thickness of the deposits (and the temperature of the detergent solution). Heat exchangers encrusted with coagulated protein must be exposed to circulating an alkaline detergent followed by an acid solution for a total of 20 – 60 minutes, whereas 10 minutes’ treatment with an alkaline solution is enough to dissolve the film on the walls of a milk tank.
Rinsing with clean water
After cleaning with detergent, the surfaces must be flushed with water long enough to remove all traces of the detergent. Any detergent left in the system after cleaning can contaminate the milk. All parts of the system must be thoroughly drained after rinsing.
Softened water is preferred for rinsing. This prevents the deposition of limescale on the cleaned surfaces. Hard water with a high content of calcium salts must therefore be softened in ion exchange filters to 2 – 4 °dH (German degrees of hardness).
The equipment and pipe systems are practically sterile after treatment with strong alkaline and acid solutions at a high temperature. It is then necessary to prevent the overnight growth of bacteria in the residual rinsing water in the system. This can be done by acidifying the final rinse water to a pH of less than 5 by adding phosphoric or citric acid. This acidic environment prevents the growth of most bacteria.
Disinfection
Properly carried out cleaning with acid and alkaline detergents renders the equipment not only physically and chemically but also, to a large extent, bacteriologically clean.
The bacteriological cleaning effect can be further improved by disinfection. This leaves the equipment virtually free from bacteria. For certain products (e.g. UHT milk), it is necessary to sterilise the equipment to render its surfaces completely free from bacteria
Dairy equipment can be disinfected in the following ways:
- Thermal disinfection (boiling water, hot water, steam)
- Chemical disinfection (chlorine, acids, iodophors, hydrogen peroxide, etc.)
Disinfection can be done in the morning, immediately before milk processing begins. The milk can be admitted as soon as all the disinfectant has been drained from the system.
If disinfection takes place at the end of the day, the disinfectant solution should be flushed out with water to avoid leaving any residues that may attack the metal surfaces.
Cleaning-in-place systems
Cleaning-in-place means that rinsing water and detergent solutions are circulated through tanks, pipes and process lines without the equipment having to be dismantled. CIP can be defined as the circulation of cleaning liquids through machines and other equipment in a cleaning circuit. The passage of the high-velocity flow of liquids over the equipment surfaces generates a mechanical scouring effect that dislodges dirt deposits. This only applies to the flow in pipes, heat exchangers, pumps, valves, separators, etc.
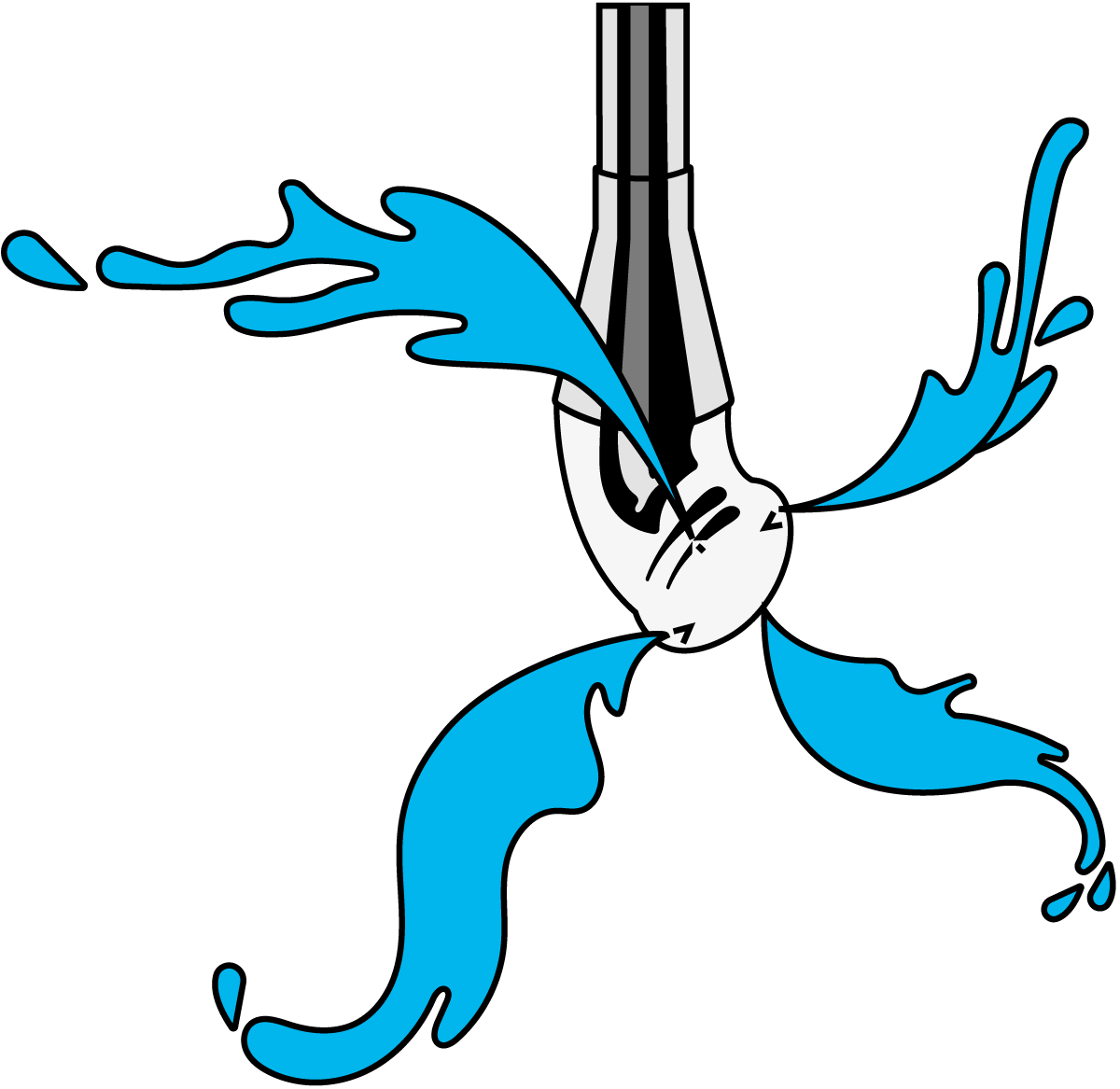
The normal technique for cleaning large tanks is to spray the detergent on the upper surfaces and then allow it to run down the walls. The mechanical scouring effect is then often insufficient, but the effect can to some extent be improved by the use of specially designed cleaning devices, one of which is shown in Figure 22.3. Tank cleaning requires large volumes of detergent, which must be circulated rapidly.
CIP circuits
The question of the type of equipment that can be cleaned in the same circuit is determined according to the following factors:
- The product residue deposits must be of the same type so that the same detergents and disinfectants can be used
- The surfaces of the equipment to be cleaned must be of the same material or materials compatible with the same detergent and disinfectant
- All components in the circuit must be available for cleaning at the same time
- The risk of contamination can be reduced by using a separate cleaning station for aseptic equipment as for upstream equipment
Dairy installations are therefore divided for cleaning purposes into a number of circuits that can be cleaned at different times.
Compatible materials and system design
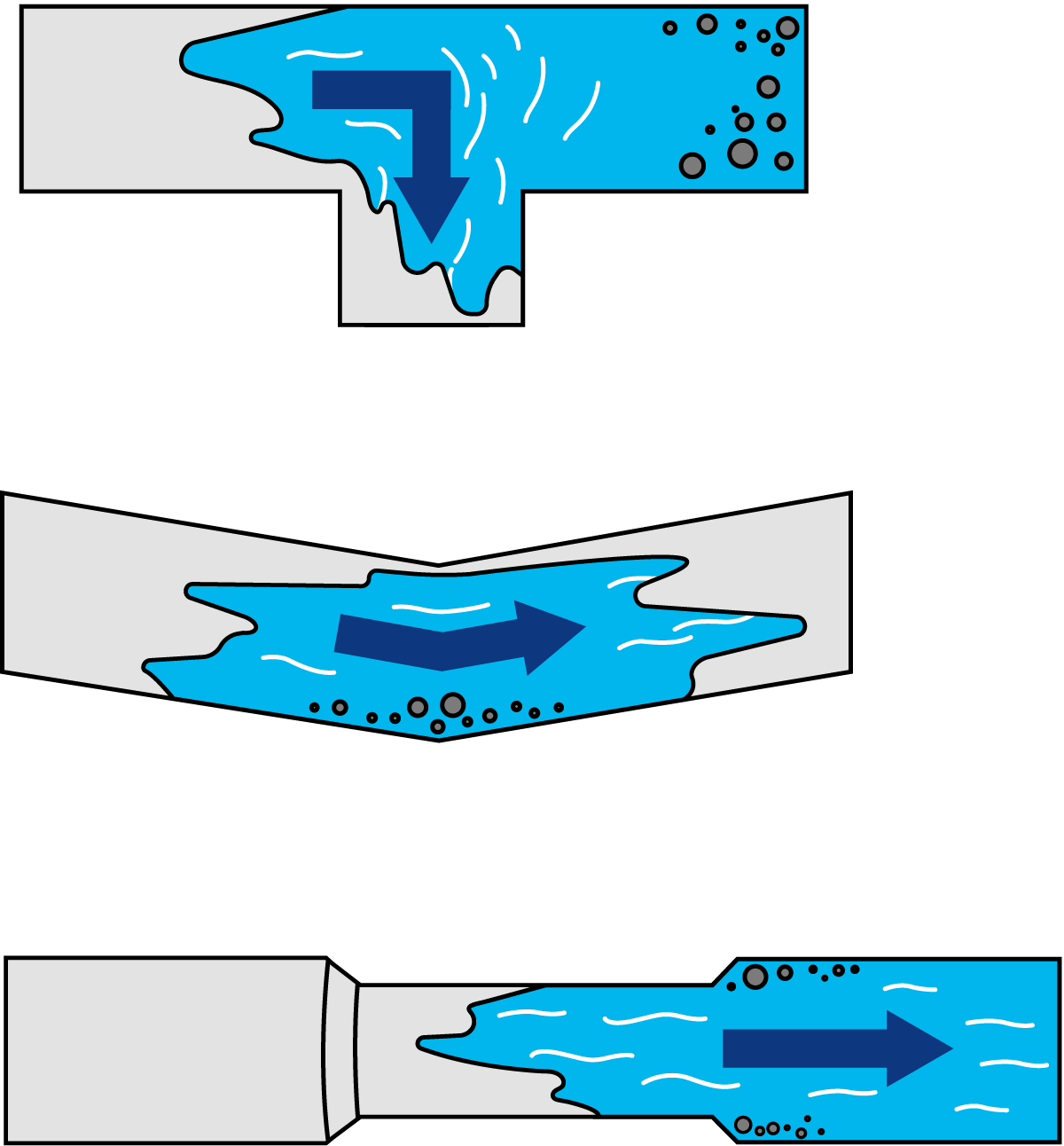
For effective CIP, the equipment must be designed to fit into a cleaning circuit and must also be easy to clean. All surfaces must be accessible to the detergent solution. There must be no dead ends that the detergent cannot reach or through which it cannot flow (Figure 22.4). Machines and pipes must be installed in such a way that they can be efficiently drained. Any pockets or traps from which residual water cannot drain will provide sites for the rapid multiplication of bacteria and cause a serious risk of infecting the product.
Materials in process equipment, such as stainless steel, plastics and elastomers, must be of such quality that they do not transmit any odour or taste to the product. They must also be capable of withstanding contact with detergents and disinfectants at the cleaning temperatures.
Stainless steel is the universal material for product-wetted surfaces in modern dairies. Dairy products don’t react with the equipment surfaces, so metallic contamination is normally not a problem. Stainless steel can, however, be attacked by chlorine solutions.
Elastomers (e.g. rubber gaskets) can be attacked by chlorine and oxidising agents, which cause them to blacken or crack and release rubber particles into the milk.
Various types of plastic in process equipment may present a contamination hazard. Some of the constituents of some types of plastics can be dissolved by the fat in milk. Detergent solutions can have the same effect. Plastic materials for use in dairies must therefore satisfy certain criteria regarding composition and stability.
CIP programmes
Dairy CIP programmes differ according to whether the circuit to be cleaned contains heated surfaces or not. We distinguish between:
- CIP programmes for circuits with pasteurizers and other equipment with heated surfaces (UHT, etc.)
- CIP programmes for circuits with pipe systems, tanks and other process equipment with no heated surfaces
Steps for CIP cleaning of 'cold' components:
- Rinsing with water
- Circulation of alkaline detergent
- Rinsing with water
- Disinfection with hot water
- Cooling with tap water
The main difference between the two types is that acid circulation must always be included in the first type to remove encrusted protein and salts from the surfaces of heat-treatment equipment.
A CIP programme for a circuit with a pasteurizer and/ or other hot components can consist of the following stages:
- Rinsing with warm water for about 10 minutes
- Circulation of an alkaline detergent solution (0.5 – 2%) for about 30 minutes at 75 °C
- Rinsing out alkaline detergent with warm water for about five minutes
- Circulation of acid solution (0.5 – 1.5%) for about 20 minutes at 70 °C
- Post-rinsing with cold water
- Gradual cooling with cold water for about eight minutes
The pasteurizer is usually disinfected in the morning before production starts. This is typically done by circulating hot water at 90 – 95 °C for 10 – 15 minutes after the returning temperature is at least 85 °C.
A CIP programme for a circuit with pipes, tanks and other "cold" components can comprise the following stages:
- Rinsing with warm water for three minutes
- Circulation of a 0.5 – 1.5% alkaline detergent at 75 °C for about 10 minutes
- Rinsing with warm water for about three minutes
- Disinfection with hot water 90 – 95 °C for five minutes
- Gradual cooling with cold tap water for about 10 minutes (normally no cooling for tanks)
Design of CIP systems
In practice, there is no limitation to satisfy stringent individual demands as to the size and complexity of CIP plants.
The CIP station in a dairy consists of all necessary equipment for storage, monitoring and distribution of cleaning fluids to the various CIP circuits. The exact design of the station is determined by many factors, including:
- How many individual CIP circuits are to be served from the station? How many are "hot" and how many are "cold"?
- Are the milk rinses to be collected? Are they to be processed (e.g. evaporation or filtration)?
- What method of disinfection is to be used? Chemical, steam or hot water?
- Will the detergent solutions be used only once or recovered for reuse?
- What is the estimated steam demand, momentary and total, for cleaning and sterilisation?
Looking back over the history of CIP, we find two schools of thought:
- Centralised cleaning
- Decentralised cleaning
Until the end of the 1950s, cleaning was decentralised. The cleaning equipment was located in the dairy, close to the process equipment. Detergents were mixed by hand to the required concentration – an unpleasant and hazardous procedure for the personnel involved. Detergent consumption was high, which made cleaning expensive.
The centralised CIP system was developed during the 1960s and 1970s. A central CIP station was installed in the dairy. Rinsing water, heated detergent solutions and hot water were supplied from this unit by a network of pipes to all the CIP circuits in the dairy. The used solutions were then pumped back to the central station, and from there to the respective collection tanks. Detergents recovered in this way could be topped up to the correct concentration and reused until they were too dirty and had to be discarded.
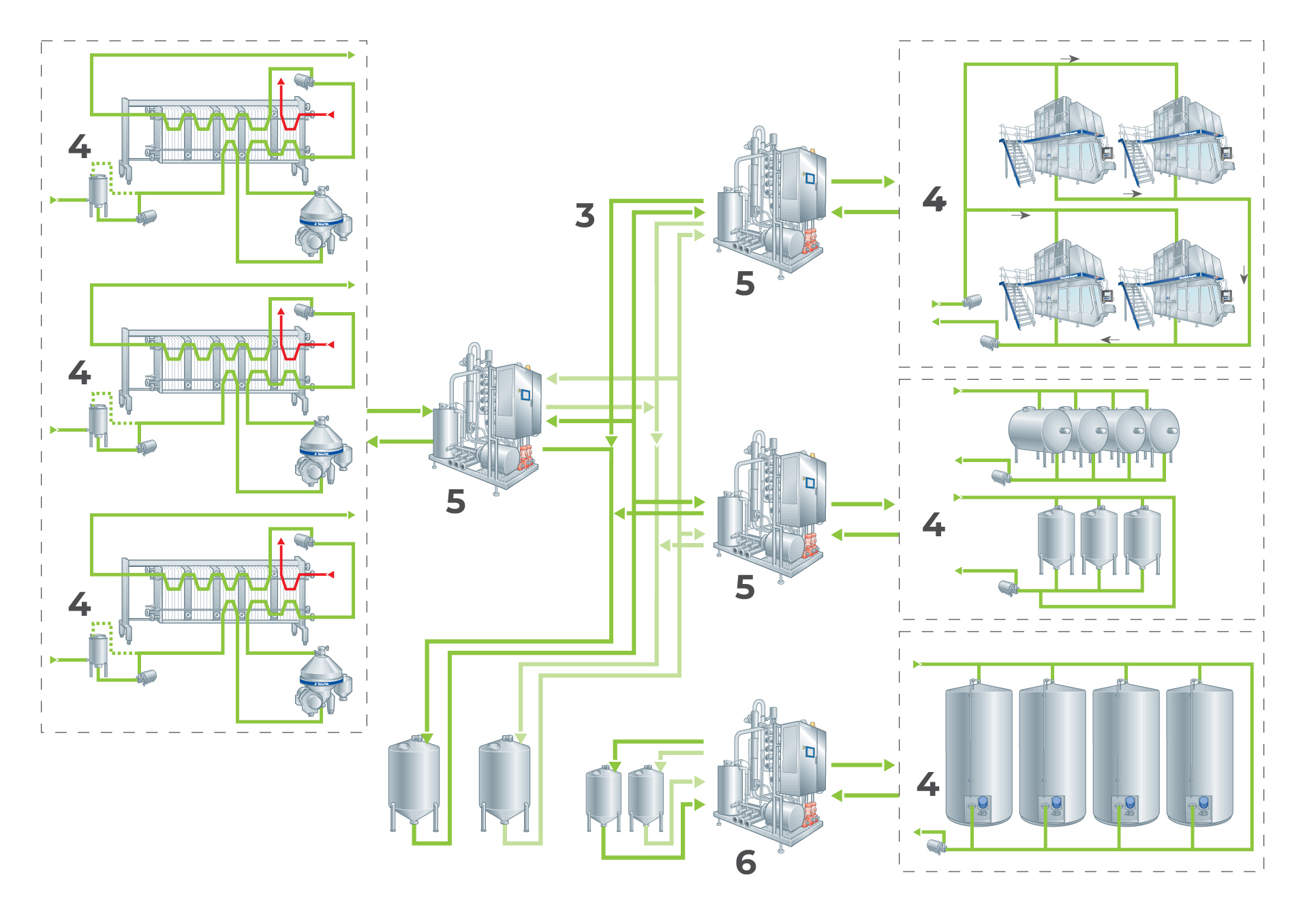
Centralised CIP works well in many dairies, but in large dairies, the communication lines between the central CIP station and the peripheral CIP circuits have grown excessively long. The CIP pipe systems contain large volumes of liquids, even when they are “drained”. The water remaining in the pipes after pre-rinsing dilutes the detergent solution, which means that large amounts of concentrated detergent must be added to maintain the correct concentration. The greater the distance, the greater the cleaning cost. A move back towards decentralised CIP stations therefore began in large dairies at the end of the 1970s. Each department had its own CIP station. Examples of the two systems are shown below.
Centralised CIP
Centralised systems are used mainly in small dairy plants with relatively short communication lines. An example is shown in Figure 22.5.
Water and detergent solutions are pumped from storage tanks in the central station to various CIP circuits.
The detergent solutions and hot water are kept hot in insulated tanks. The required temperatures are maintained by heat exchangers. The final rinse water is collected in a rinse-water tank and used as pre-rinsing water in the next cleaning programme. The milk/water mixture from the first rinsing water is collected in a rinse-milk tank.
The detergent solutions must be discharged when they have become dirty after repeated use. The storage tank must then be cleaned and refilled with fresh solutions. It is also important to empty and clean the water tanks, especially the rinse-water tank, at regular intervals to avoid the risk of infecting an otherwise clean process line.
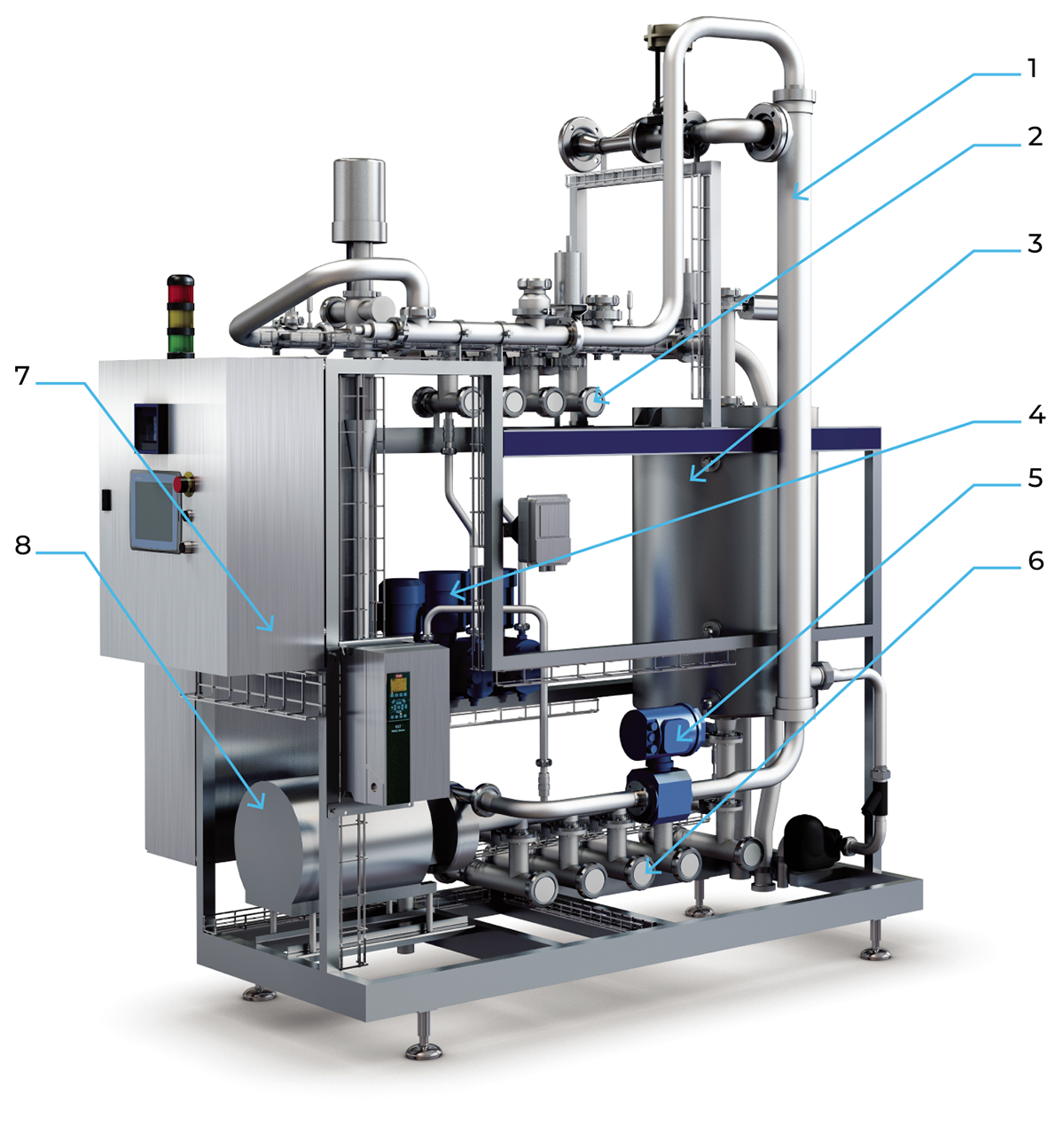
An example of the design of a central CIP station is illustrated in Figure 22.6.
A station of this type is usually highly automated. The tanks have electrodes for high- and low-level monitoring. Returning of the cleaning solutions is controlled by conductivity transmitters. The conductivity is proportional to the concentrations normally used during dairy cleaning. At the phase of flushing with water, the concentration of detergent solution becomes lower and lower. At a pre-set value, a change-over valve routes the liquid into the drain, instead of the relevant detergent tank. CIP programmes are controlled by a computerised sequence controller. Large CIP stations can be equipped with multiple tanks to provide the necessary capacity.
Decentralised CIP
Decentralised CIP is an attractive alternative for large dairies, where the distance between a centrally located CIP station and peripheral CIP circuits would be extremely long. The large CIP station is replaced by a number of smaller units located close to the various groups of process equipment in the dairy.
Figure 22.7 illustrates the principle of a decentralised CIP system, also called a satellite CIP system.
This still has a central station for storage of the alkaline and acid detergents, which are individually distributed to the individual CIP units in the main lines. Supply and heating of rinsing water (and detergent, when required) are arranged locally at the satellite stations, one of which is shown in Figure 22.8.
These stations operate on the principle that the various stages of the cleaning programme are carried out with a carefully measured minimum volume of liquid – just enough to fill the circuit to be cleaned. A powerful circulation pump is used to force the detergent through the circuit at a high flow rate.
The principle of circulating small batches of cleaning solutions has many advantages. Water and steam consumption, both momentary and total, can be greatly reduced. Milk residues from the first rinse are obtained in a more concentrated form and are therefore easier to handle and cheaper to evaporate. Decentralised CIP reduces the load on sewage systems as compared to centralised CIP, which uses large volumes of liquid.
The concept of single-use detergents has been introduced in conjunction with decentralised CIP, as opposed to the standard practice of detergent recycling in centralised systems. The one-time concept is based on the assumption that the composition of the detergent solution can be optimised for a certain circuit. The solution is considered spent after having been used once. In some cases, however, it may be used for pre-rinsing in a subsequent programme.
Verifying the cleaning effect
Verification of the effect of cleaning must be regarded as an essential part of cleaning operations. As there are presently no techniques available for measuring cleanliness continuously in line, a plant must be opened after cleaning at predetermined critical control points to assess cleanliness in one or more ways:
- It must be visually clean without any product residues. This can be checked with a clean white cloth.
- Cleanliness can be tested microbiologically by swabbing specific areas. This test requires time for incubation and analysis.
- A quicker method is to test for the presence of Adenosine triphosphate (ATP) using an enzyme that emits light when in contact with ATP. The level of luminescence can then be measured, proving the presence of a living organism, or of a substance produced by a living organism.
Apart from verifying the effect of cleaning, critical CIP parameters (flow rate, temperature, time, electrical conductivity) should be monitored and recorded during the cleaning to ensure that the programme has run according to the chosen conditions. Lastly, cleaning validation ensures that a producer is systematically following good cleaning regimens that consistently produce an acceptable result, minimising the risk of spoiled products. A structured method to validate cleaning must accomplish two things:
- It verifies the effectiveness of the cleaning procedure for the removal of product residues.
- It documents evidence that the cleaning process removes residues to predetermined acceptable levels, repeatedly and reliably.