MILK AND WHEY POWDERED INGREDIENTS

Drying serves as a method of preserving food, inhibiting the development of microorganisms, such as yeasts and moulds, by extracting water. It stands as one of humanity’s oldest preservation techniques, with roots tracing back to ancient times. According to Marco Polo’s travel accounts in Asia, Mongolians practised sun-drying milk to produce milk powder. Nowadays many different types of products are prepared by dehydration using dryers across various industries including chemical, pharmaceutical, processing, and dairy. Dehydrated food products encompass a diverse range of fruits, meats, dairy products like milk and whey, and various types of baby food. Reducing water content can be achieved through different methods. To accomplish this, the industry offers a variety of dryer types, with selection depending upon the desired characteristics of the final product.
Drying of foods
Introduction
There are two main reasons for drying food:
- To prevent or inhibit the growth and activity of micro-organisms, thereby preserving the food
- To reduce the weight and bulk of food products, making transportation and storage more economical
When drying is performed correctly, rehydrated dried foods retain nearly the same nutritional quality, colour, flavour, and texture as fresh food. However, improper drying can lead to significant loss in nutritional and sensory qualities, and more significantly, pose a risk of microbial spoilage and potential foodborne illness.
The principles of drying
Drying represents a mass transfer process wherein water or another solvent is extracted through evaporation from a solid, slurry, or liquid. Commonly employed as a final production stage, prior to product sale or packaging, this process typically involves the use of heat and a medium to expel the vapour produced. In bio-based products like food, grains, and pharmaceuticals, water is typically the solvent targeted for removal.
Traditionally, foods undergo drying using hot air to remove moisture. Optimal drying necessitates hot, dry, and moving air. These factors are interrelated, and each aspect needs to be appropriately balanced. Cold moving air or hot and humid air for example are both inadequate. Air dryness is quantified as humidity – the lower the humidity, the dryer the air. Humidity is often expressed as the ratio of water vapour in the air to fully saturated air, known as relative humidity (RH). Completely dry air exhibits an RH of 0%, while air saturated with water vapour represents an RH of 100%.
Air with a low relative humidity has the capacity to pick up and hold more water than air with a high relative humidity at the same temperature. The principle of drying is that hot and dry air is mixed with liquid food and absorbs the moisture from the food. This saturated air then must be replaced with dry air so that the process of extracting moisture from the food can continue until the food is dry. If wet air with a high relative humidity is used, i.e. in tropical climates, it quickly becomes saturated and cannot pick up the same amount of moisture from the food. The drying process in the humid tropics therefore takes longer than in the semi-arid tropics.
The medium that is used for drying is typically ambient air which is cleaned and heated to provide the required energy to evaporate the water in the food. Water is evaporated under adiabatic conditions, meaning that the heat from the air is used to transform the water into vapor absorbed by the drying air.
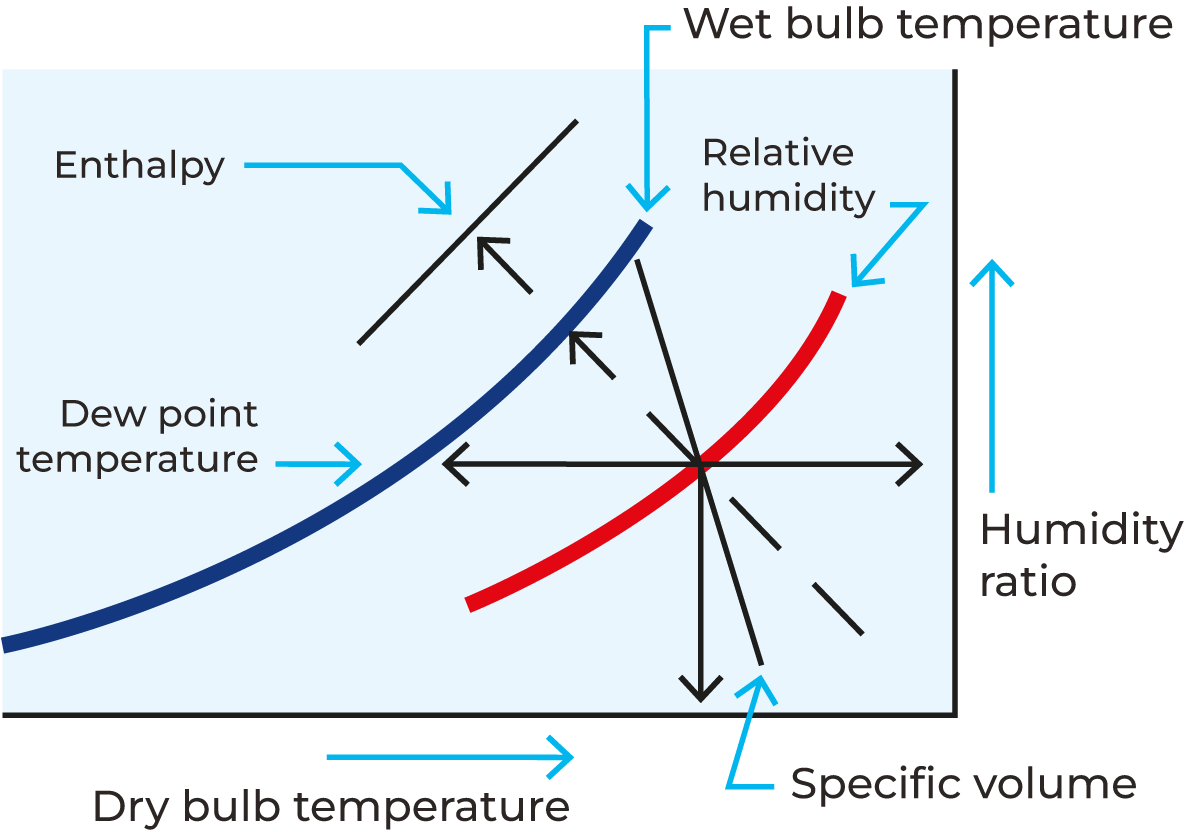
The temperature of the air affects the humidity – higher temperatures reduce the humidity and allow the air to carry more water vapour. The relationship between temperature and RH is shown on a psychrometric chart (see Figure 19.1).
The different terms describing the conditions of the drying air are the following:
- Dry bulb temperature: The temperature of unsaturated air measured by a thermometer freely exposed to the air but shielded from radiation
- Wet bulb temperature: The adiabatic saturated temperature indicated by a thermometer covered in a water-soaked cloth (wet bulb) over which air is passed. As the water evaporates from the cloth, it cools the thermometer. The wet-bulb temperature reflects the combined effect of air temperature and humidity. By combining the dry bulb and wet bulb temperature in a psychrometric chart or Mollier diagram, the state of the humid air can be determined
- Dew point: The temperature where water vapour starts to condense out of the air (the temperature at which air becomes completely saturated). Above this temperature, the moisture stays in the air. If the dew-point temperature is close to the dry air temperature, the relative humidity is high. If the dew point is well below the dry air temperature, the relative humidity is low.
- Absolute humidity: The actual amount of water vapour (moisture) in the air expressed in kg per kg of dry air, regardless of the air’s temperature.
- Relative humidity: The relationship between the moisture content of air at a certain temperature and the moisture content of saturated air at the same temperature. Relative humidity (RH) is given as a percentage from 0 – 100. 100% RH means that the air is fully saturated with moisture, and the dew point has been reached.
- Drying air rate: The flow rate of air used in a drying process to remove moisture from a substance or material. It is typically measured in terms of volume or mass per unit of time such as cubic meters per hour (m³/h) or kilogram per hour (kg/h).
- Heat capacity: The heat capacity of air refers to the amount of heat energy required to raise the temperature of a specific quantity of air by one degree Celsius (or Kelvin). It is typically measured in units of energy per unit mass per degree Celsius (or Kelvin), such as joules per kilogram per degree Celsius (J/kg °C).
The use of a psychrometric chart
A psychrometric chart for air (Mollier diagram or h-x diagram) is a graphical representation of the thermodynamic properties of moist air. It typically displays parameters such as wet and dry bulb temperature, humidity ratio, relative humidity, specific volume, enthalpy, and dew point temperature. These charts are essential tools for engineers and scientists working in fields related to heating, cooling, (de)humidification, and drying processes. By using a psychrometric chart, engineers can analyse and understand the behaviour of moist air under different conditions. Nowadays software applications are available to calculate the characteristics of air. Figure 19.1 shows a simple version of a psychrometric chart.
Rate of drying
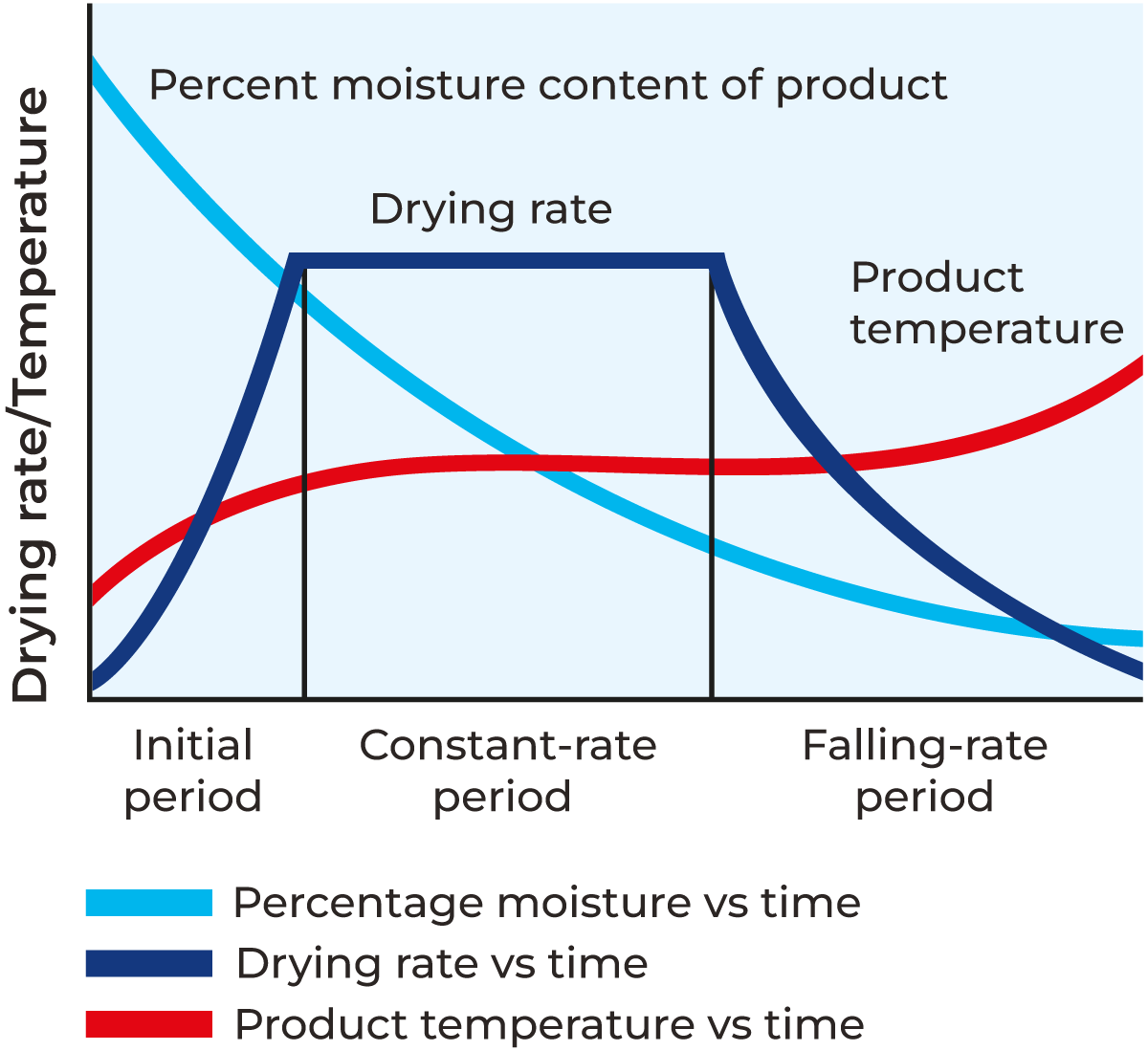
Drying of food is defined in three distinct phases, the initial period, the constant-rate period, and the falling-rate period. In the initial period, the rate of drying increases with time, and surface water and interstitial water evaporate. In the constant rate period, the rate of drying remains constant with time and free water evaporates. In the falling rate period, the rate of drying decreases with time. As drying proceeds, water must be removed from the inside of the food. This becomes increasingly difficult as the water activity decreases, and the water must migrate further through the food from the centre to the outside from where it is evaporated. The rate of heat transfer exceeds that of mass transfer and the particle begins to heat up faster. Eventually, no more moisture can be removed from the food, and it is said to be in equilibrium with the drying air. In all periods the percentage of moisture decreases exponentially while the product temperature increases.
During the falling rate period, the rate of drying is mainly controlled by the chemical composition and physical structure of the food. The temperature of the drying air is equally important during this phase as hot air helps the moisture inside the food to move towards the surface.
Throughout the evaporation process, the temperature of the droplets ranges between that of the surrounding air and its wet bulb temperature. The moisture content of the droplet dictates its water activity (Aw), which, along with the relative humidity of the surrounding air, determines the actual temperature of the droplet/particle within this range. As evaporation progresses, the water activity gradually decreases, leading to a gradual increase in particle temperature towards that of the surrounding air. Upon reaching equilibrium with the drying air, the particle’s water activity matches the relative humidity of the surrounding air, resulting in the particle temperature being equivalent to the surrounding air temperature. This temperature profile ensures that products do not get overheated during drying which, in turn will preserve their quality. Figure 19.2 shows the different drying phases for drying.
The importance of droplet and particle size
When milk is spray dried, heat and mass transfer occur at exceptionally high rates within a very short time frame. The main factor that controls the drying rate is the rate at which moisture can move from the inside of a droplet of food to the surface. Therefore, the shorter the distance that moisture must travel, the faster the drying rate. For this reason, wherever possible, the atomised product should be reduced to small droplets prior to drying. Reducing the size also increases the surface area of the food in relation to the volume of the droplets. This in turn increases the rate at which water can be evaporated from the food.
However, a too-high drying rate may inhibit the migration of moisture from the centre to the surface of the droplets resulting in so-called case hardening. Case hardening in the context of spray drying refers to the phenomenon where a dried crust or shell forms on the surface of droplets before the interior has fully dried. This can lead to issues in the final product, such as incomplete drying, uneven moisture content, and potential quality degradation. Case hardening can be prevented by using relatively colder drying air.
Storage and stability of dried foods
To ensure safe storage of dried food, the final moisture content should be less than 20% for fruits and meat, less than 10% for vegetables, and 2.5 – 5.0% for dairy products. The stability of a dried food during storage depends on its moisture content and the ease at which the food can pick up moisture from the air. The risk of moisture pick-up is of course greater in regions of high humidity. However, different foods absorb moisture to different extents. For foods that readily pick up moisture, it is necessary to package them in a non-permeable, moisture-proof material.
Low moisture content is only an indication of food stability and not a guarantee. It is the availability of moisture for microbial growth that is more important, and the term water activity (Aw) is used to describe this. Water activity (Aw) is a measure of the availability of water in a substance, typically in food or pharmaceuticals, for chemical reactions and microbial growth. It is defined as the ratio of the vapour pressure of water in a substance to the vapour pressure of pure water at the same temperature. The value of water activity ranges from 0 (completely dry) to 1 (pure water).
Importance of water activity in food:
- Safety: Foods with high water activity need more preservation methods to prevent microbial growth.
- Shelf life: Lower water activity generally means a longer shelf life because fewer microorganisms can survive.
- Quality: Proper control of water activity can maintain the desired texture, taste, and appearance of food products.
By understanding the water activity of different foods, manufacturers can design better preservation and packaging methods to ensure the safety and quality of their products.
Examples of moisture contents and Aw values for selected foods and their packaging requirements are listed in Table 19.1.
Drying methods
There are many different methods for drying foodstuffs, each with their own advantages for different applications; these include, amongst others:
- Drum or roller-drying
- Freeze drying
- Spray drying
- Shelf drying
- Bed drying
- Supercritical drying
- Dielectric drying
- Etc.
Commonly used drying methods are explained below:
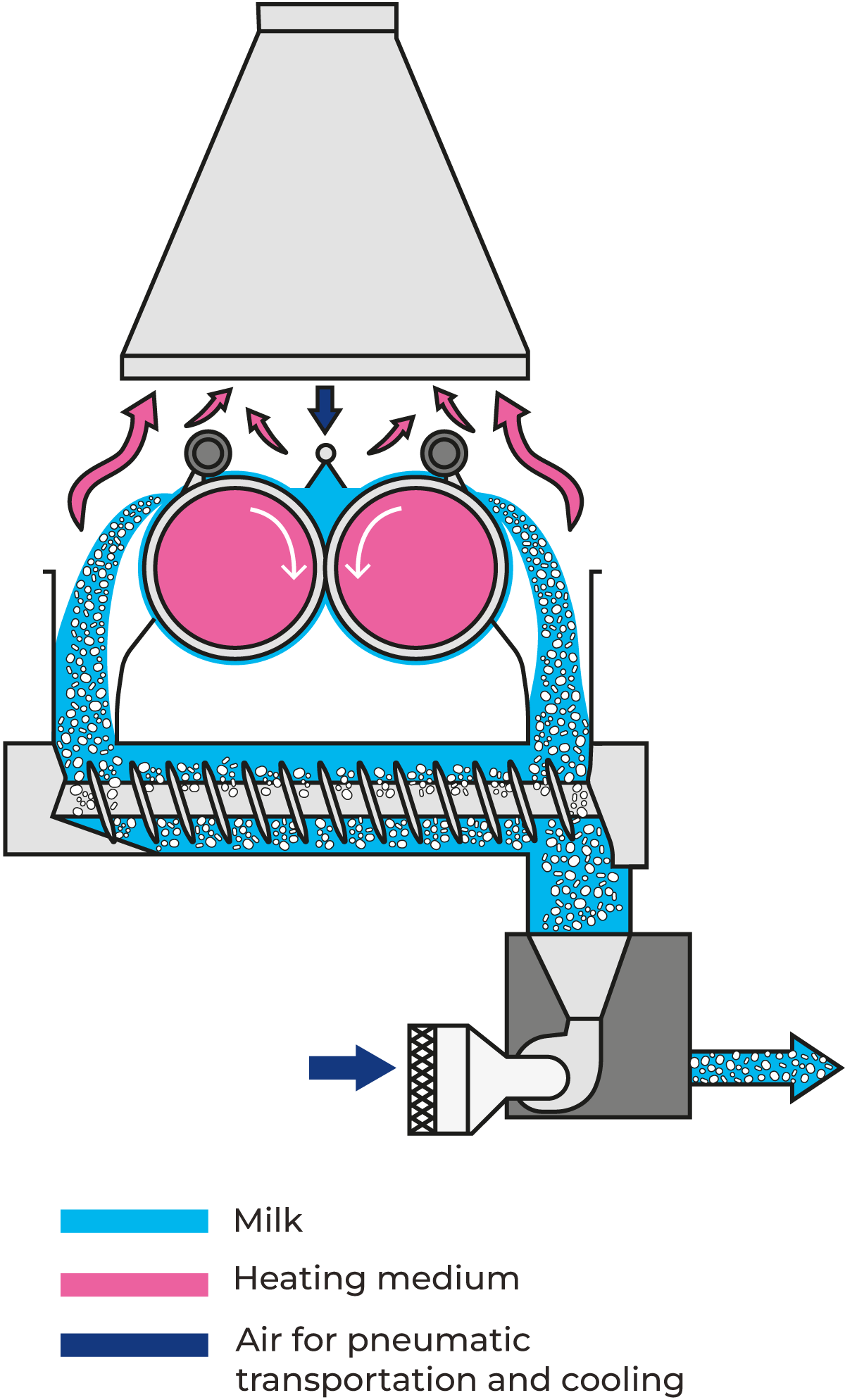
Drum or roller drying
Drum or roller drying is a method used for drying liquid products. In the drum-drying process, the material is dried at relatively low temperatures over rotating, high-capacity, steam-heated drums that produce sheets of drum-dried product. The water in the concentrate evaporates and the vapour is drawn off. This product is milled to a finished flake or powder form.
Depending on the desired capacity, the roller dryer is 1 – 6 m long and has a diameter of 0.6 – 3 m. Its performance is dependent on film thickness, roller surface temperature, roller speed and the dry matter content of the supplied product.
Modern drum drying techniques result in dried ingredients which reconstitute immediately and retain much of their original flavour, colour and nutritional value. The relatively high temperature of the heating surfaces may denature the proteins in milk, resulting in deterioration of solubility and brown discolouration. On the other hand, this intensive heat treatment increases the water-binding properties of the powder.
Some advantages of drum drying include the ability to dry viscous foods which cannot be easily dried with other methods. Other products where drum drying can be used are, for example starches, breakfast cereals, and instant mashed potatoes to make them cold-water soluble. Drum dryers are not used often for milk products.
Freeze drying
Freeze drying, also known as lyophilisation or cryodesiccation, is a dehydration process typically used to preserve delicate food. It involves freezing the material and then removing the ice (water) by sublimation under vacuum, leaving behind a dried product.
The material to be dried is first frozen to very low temperatures. This freezing step is crucial, as it helps in forming ice crystals within the material.
The frozen material is placed in a vacuum chamber to lower the pressure. This reduced pressure allows the ice to sublime directly from solid to vapour without passing through the liquid phase. Sublimation is facilitated by providing heat to the material, usually using heated shelves or plates. The heat energy breaks the bonds between the ice crystals and facilitates the transition to vapour.
After the freeze-drying process is completed, the vacuum is usually broken by means of an inert gas such as nitrogen before the material is packed and sealed. Freeze-drying is not widely used to produce milk powder because of the high energy demand.
Spray drying
Spray drying is a widely used industrial process for converting liquid or slurry materials into dry powders. This process is particularly valuable in the food, pharmaceutical, and chemical industries. Spray drying involves atomising a liquid into a spray of fine droplets which are then exposed to hot gases for rapid drying to produce a dry powder. Spray drying is very suitable for the continuous production of thermally sensitive products from liquid feedstocks due to the low thermal exposure of the particles. The technology is ideal when the end product must comply with defined quality standards. This regards particle size distribution, residual moisture content, bulk density, flowability, dissolvability and many other physical and functional properties.
Spray drying
Introduction
Spray drying is an effective method for preserving valuable nutritional ingredients in a powdered form, despite its high energy consumption and the size of the spray dryer equipment and building required. To minimise energy costs, the solids in the liquid feed to be dried are maximised through water removal by membrane filtration and/or evaporation, which only requires about 10 to 12% of the energy used for spray drying. Without prior concentration, the powder particles also would be very small, possessing high air content, inadequate wettability, and a limited shelf life.
Spray-dried products vary in composition, applications, properties, and functionalities, necessitating flexible spray dryers to meet various demands. Typically, spray drying is the final stage in the manufacturing process, and the end products are used in various ways in the market. Powdered products are often easier to handle, store, and transport compared to liquid counterparts. Therefore, the spray dryer is a crucial unit operation in manufacturing plants that utilise spray drying.
Principles of spray drying
Powder production in a spray dryer is carried out in multiple phases:
- Atomisation of the concentrate into very fine droplets mixed with hot air
- Water evaporation/drying
- Separation of the powder from the drying air
- Post-treatment of the powder to meet the required properties
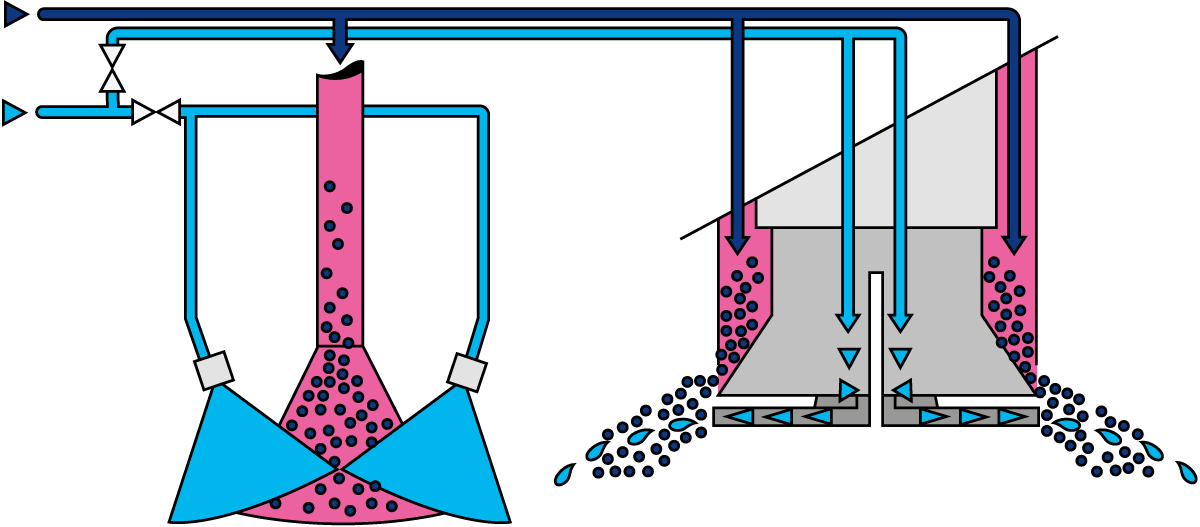
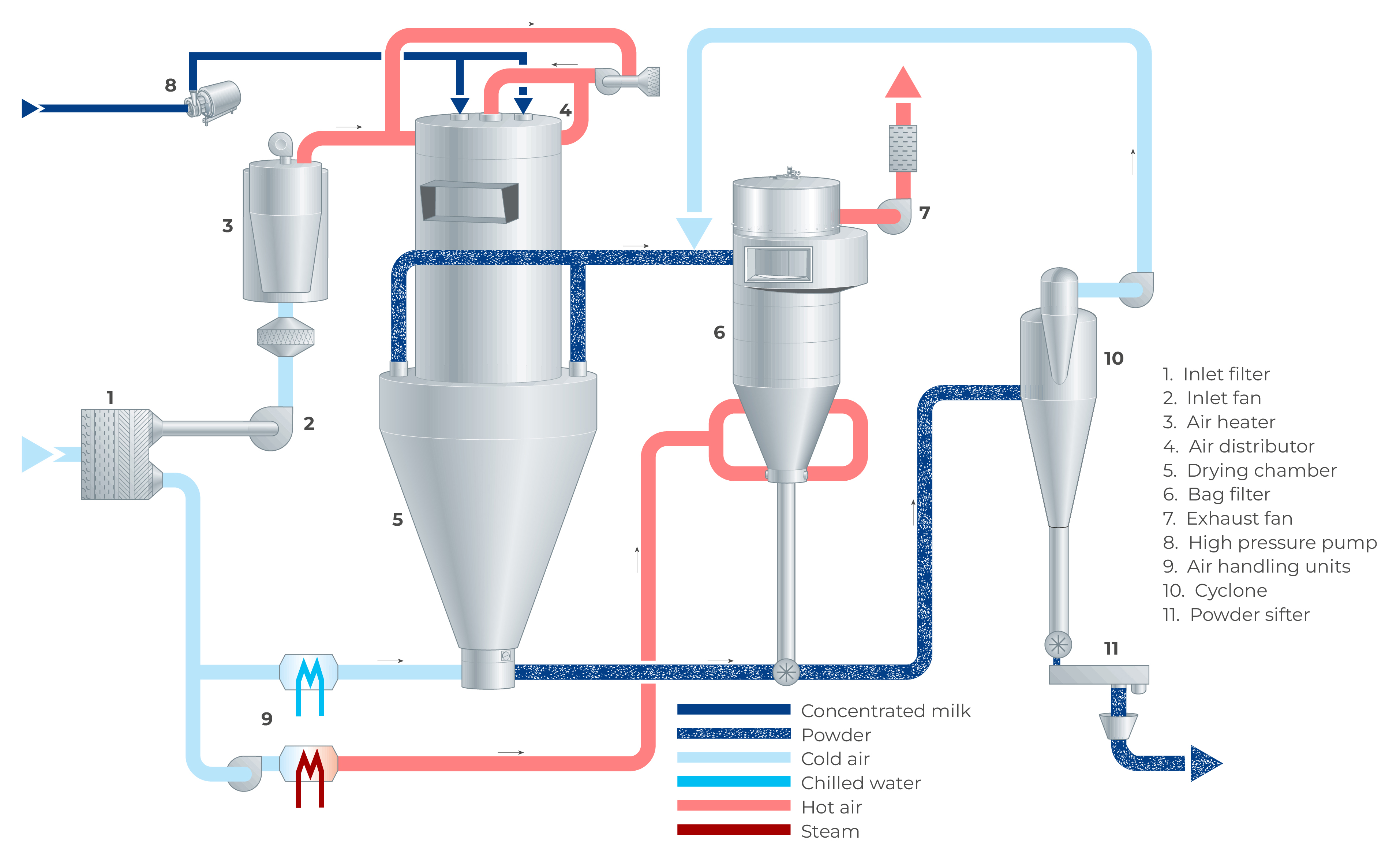
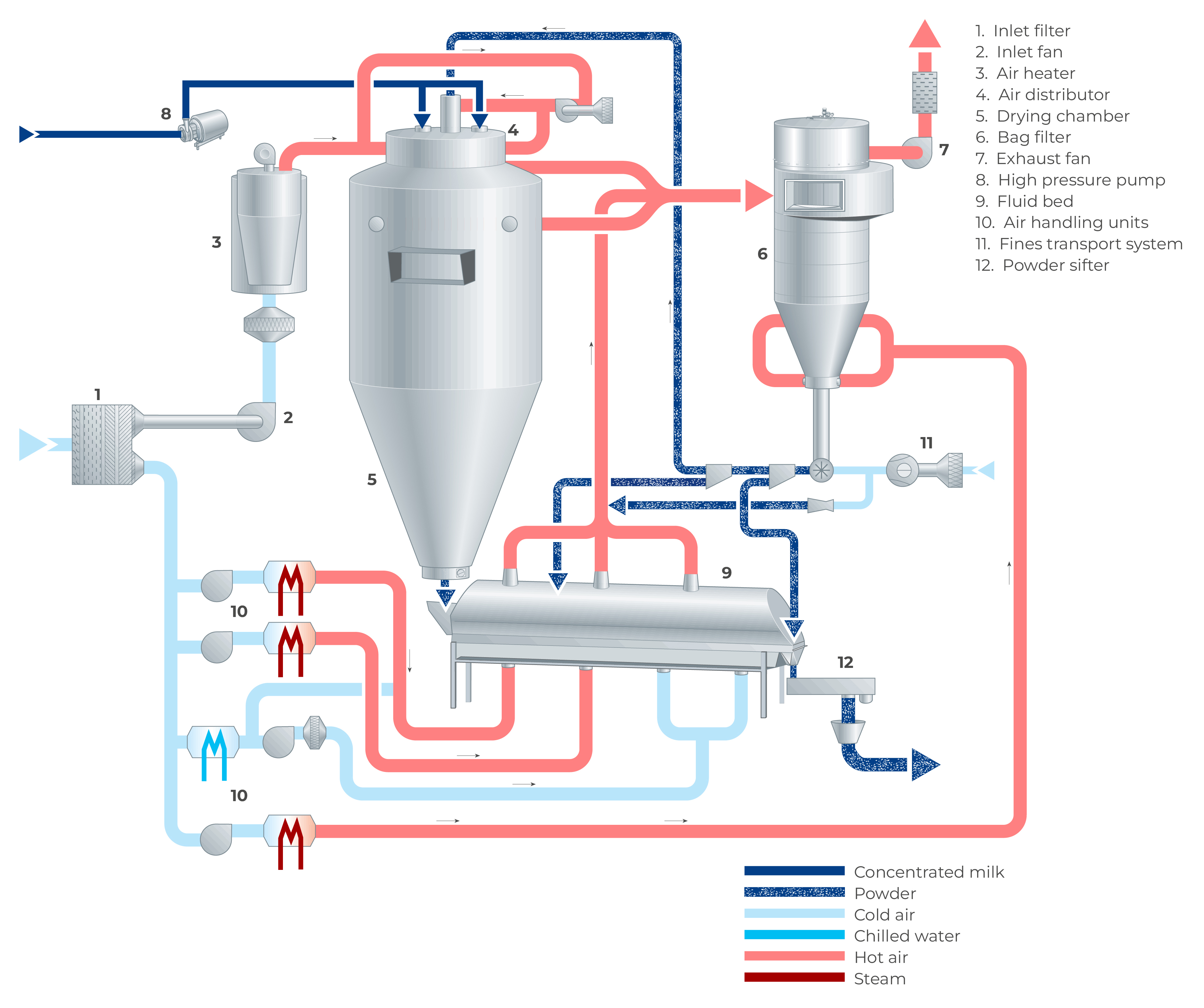
In a spray dryer, water from the liquid feed to be spray dried is evaporated from the surface of many small droplets (1 litre of concentrate is atomised to 1.2 x 1011 droplets with a diameter of 50 microns with a total surface of 120 m2). The fine mist of droplets formed by an atomiser is introduced into a hot air stream which is cooled down due to the rapid evaporation of the water from the liquid feed. After atomisation, the spray droplet cools down instantly to the wet bulb temperature, which is typically approximately 50 ºC. The colder and more humid exhaust air is discharged from the dryer into the atmosphere after the separation of the dry particles. The air for drying is usually heated to temperatures between 150 – 300 °C, depending on the material being dried. Powder which is formed in the drying chamber is continuously discharged at the bottom of the chamber while the finer particles are recovered from the exhaust gases using cyclones, bag filters or a combination of the two. The powder is further treated in downstream equipment to obtain the final moisture content, temperature, and other functional properties. For post-treatment of the product, fluidising beds are often used. Figure 19.4 shows an example of a spray dryer system.
Depending on the specific product being dried, either single-stage or multi-stage drying methods may be employed. In single-stage drying, the final product moisture content is attained within the drying chamber. In multi-stage drying, additional water removal occurs downstream of the dryer in, for instance, fluidising beds.
Justifications for utilising spray drying include:
- Water removal efficiency: Spray drying facilitates a significant reduction in volume and weight through water removal, leading to substantial savings in packaging materials and energy consumption. This reduction in energy usage can be attributed to the efficiency of spray drying.
- Preservation of perishable ingredients: By lowering the water activity of dried materials to levels where bacteria, moulds, and yeasts cannot proliferate, spray drying effectively preserves perishable foods and other ingredients, allowing for extended shelf life.
- Mild temperature processing: Spray drying is a relatively gentle process in terms of temperature, minimising damage to heat-sensitive constituents present in the product.
- Customisable heat treatments: Prior to drying, products in liquid form can undergo specific heat treatments, allowing for the creation of tailored functionalities and the elimination of microorganisms.
- Functional enhancement: Spray dryers offer versatility beyond water evaporation. Through processes like agglomeration, incorporation of dry ingredients, and coating with hydrophilic agents, products can acquire enhanced functionalities and instant properties.
- Versatile applications: Many spray-dried products serve as ingredients in dry mixtures or as fillers in tablets such as lactose, starches, and maltodextrin.
Glass transition and stickiness
The development of powder deposits in spray dryers is a well-known phenomenon caused by certain product characteristics often described with terms like “stickiness” and “thermoplasticity”.
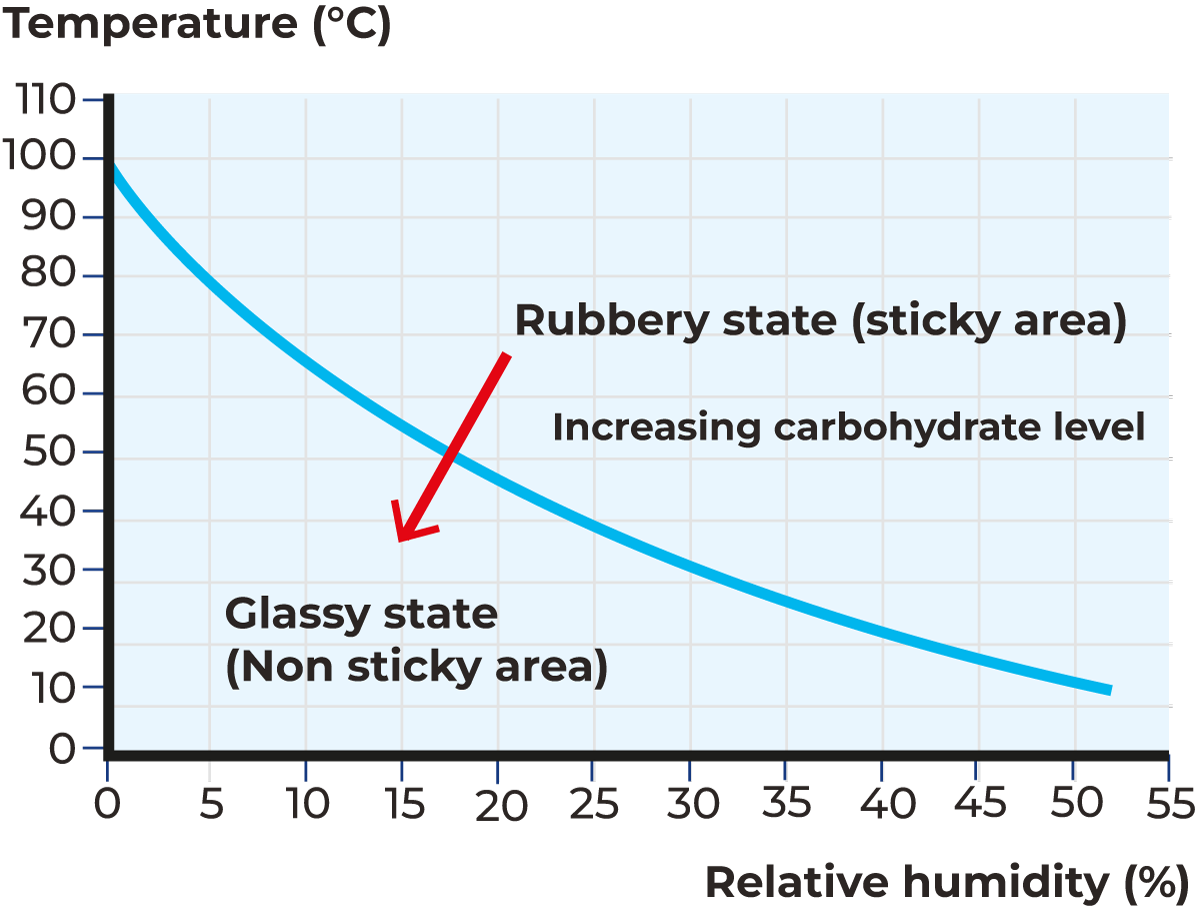
Thermoplasticity implies that the product plasticises at elevated temperatures. It is well known that increased powder moisture increases the stickiness of the products. The relation between powder moisture and the outlet temperature is also influenced by other parameters like inlet air temperature, total solids, and ambient humidity. Increased inlet temperature and/or total solids increases plant capacity, resulting in increased powder moisture and potentially powder deposits if not compensated for by adjusting the outlet air temperature. Increased ambient air humidity will have the same effect. However, the outlet temperature can only be increased within limits, partly for product quality reasons, but also because the powder particles become sticky at a certain outlet temperature due to thermoplastic behaviour. This phenomenon is shown in Figure 19.5. It should be emphasised that the sticky curve depends only on the product composition, whereas the outlet air temperature – moisture relation (and hence particle temperature – moisture relation) is dependent on product properties (composition) as well as drying conditions (T-in, TS, etc.).
There is a strong relationship between product composition, operating conditions (temperatures, humidity) and product stickiness. One of the major factors affecting stickiness during dehydration is the composition of the product in certain constituents, which are sugars, organic acids, and fats. The major sugars which cause stickiness are glucose, galactose, fructose, and sucrose. Other sugars, such as lactose, although to a lesser extent, also pose some potential problems while drying if the operating conditions are not appropriate. The presence of organic acids like citric and lactic acid leads to similar problems.
The properties of an amorphous material are determined by the extent to which the molecular motion is restricted or hindered. As the temperature falls, molecular motion is reduced. When cooled further, a transition occurs where a glassy frozen state is formed without the motion of atoms in the molecule. At this point, the material is glassy, brittle and non-sticky. Above the glass transition, it is flexible or rubbery and sticky. This boundary is known as the glass transition temperature (Tg).
In Table 19.2. the glass transition temperatures (Tgs) of various products are listed and are only valid for water-free constituents. The glass transition temperatures decrease sharply if the particle moisture increases. The significance of the Tg for spray drying is huge because the Tg has a close relation with product stickiness.
The physical state of the product changes as it passes through the dryer from solution to syrup and finally to solid form. If the viscosity of the product is below a critical level, it may stay as syrup even at low moisture levels. Depending upon the product characteristics, composition, and drying conditions, the surface of the drying droplets may remain plastic, resulting in sticking on the dryer wall or among themselves. The amorphous product obtained at the end of the drying process could, therefore, be either syrup, a sticky powder, or a relatively free-flowing powder. In a practical sense, the critical viscosity is reached at temperatures 10 – 20 °C above Tg. It can be therefore assumed that the temperature of the surface of the particle during spray drying should not reach 10 – 20 °C above the Tg. This range, however, depends on several factors and may be different per product.
Product feed
The liquid feed to be dried can be a solution, suspension, emulsion, or slurry and entails several features that are important for the performance of the spray dryer and final product quality. These include:
- Viscosity: The viscosity of the liquid feed affects atomisation and droplet formation during spray drying. Low-viscosity feeds are easier to atomise and generally result in finer particles, while high-viscosity feeds may require special atomisation techniques or equipment to achieve the desired particle size and distribution.
- Solid content: The solid content of the liquid feed, expressed as a percentage of dry matter, influences drying kinetics and powder properties. Higher solid content can lead to faster drying rates and higher powder yields but may also affect the flowability and solubility of the final product.
- Particle size distribution: The initial particle size distribution of the liquid feed impacts the size and distribution of particles in the dried powder. Controlling the particle size distribution of the liquid feed can help achieve the desired powder characteristics and properties.
- Temperature sensitivity: Some liquid feeds may contain temperature-sensitive components that can degrade or denature during spray drying. Understanding the temperature sensitivity of the feed allows for appropriate process parameters to be selected to minimise heat damage and preserve product quality.
- Chemical composition: The chemical composition of the liquid feed, including the presence of sugars, proteins, fats, and other compounds, influences the behaviour of the feed during spray drying and the properties of the resulting powder. Certain components may affect drying kinetics, particle morphology, and product stability.
- Additives or ingredients: Liquid feeds may contain additives or ingredients such as stabilisers, emulsifiers, flavours, colours, or functional ingredients. These components can affect product stability, functionality, and sensory attributes and must be considered during spray drying process design and optimisation.
Heating of the liquid feed prior to atomisation is commonly used to control the viscosity and hence the performance of the dryer and final product properties.
Atomisation
The main objective of atomising liquid feed is to provide a very large surface from which the evaporation of water can take place. Finer atomisation leads to increased specific surface area, enhancing the efficiency and effectiveness of the drying process. For instance, while one litre of milk in a spherical shape possesses a surface area of about 0.05 m2, atomising this quantity in a spray dryer results in small droplets with individual surface areas ranging from 0.05 to 0.15 mm2, thereby increasing the specific area by approximately 700 times. Table 19.3 shows the correlation between droplet size and evaporation time. The type of atomisation depends on factors like the type of product, the desired particle size and the properties required of the dried product. These may include texture, particle size, bulk density, solubility, wettability, and density.
The two predominant atomisation systems are:
- High-pressure atomisation
- Rotary disc atomisation
There is a significant functional distinction between nozzle and centrifugal atomisation methods. Figure 19.6 illustrates a stationary nozzle, which directs milk spray in the same direction as the airflow, and a centrifugal disc that directs the spray perpendicular to the airflow.
The nozzle pressure plays a crucial role in the particle size during atomisation. Higher pressures, reaching up to 300 bar result in very fine powder with high density. Conversely, lower pressures, ranging from 50 to 200 bar, yield larger particles with reduced fines content. Multi-plunger high-pressure pumps are utilised to generate these pressures.
The centrifugal atomiser comprises an electrically driven disc with multiple horizontal passages. The product is introduced into the centre of the disc and propelled through the passages at high velocity by centrifugal force. The rotational speeds of these discs typically range from 5,000 to 25,000 rpm, depending on their diameter, achieving peripheral speeds of 100 to 200 m/s. Upon exiting the passages, the product flow is atomised into fine droplets due to the high speed.
The droplet size, and consequently the particle size of the powder, can be directly influenced by adjusting the atomiser speed. A positive displacement pump is generally adequate for supplying liquid feed to this type of atomiser. While nozzle atomisation typically yields larger particle sizes ranging from 150 to 300 μm compared to 40 to 150 μm with centrifugal atomisation, the latter offers straightforward operation and is less sensitive to variations in product viscosity and supply quantity.
Drying
When the atomised concentrate contacts the hot air, the water evaporates instantly, forming powder particles. By positioning the rotary atomiser or high-pressure nozzles at the outlet of the air disperser or venturi, close contact exists between the atomised product and the drying air (initial drying). This close contact causes the water in the fine, atomised droplets to evaporate immediately, resulting in a temperature drop from approximately 200 °C to 70 – 90 °C, depending on the type of product being dried. For products with a high carbohydrate content, like infant powders, higher outlet air temperatures are typically applied.
As there is a relation between the moisture content of the powder and the relative humidity of the drying air, the concentrate supply to the atomisation system is controlled by means of the temperature of the air leaving the drying chamber. During drying, the powder settles in the drying chamber, with larger particles discharging at the bottom due to gravity. The finer powder particles, the so-called fines, will leave the drying chamber together with the drying air at the outlets. These finer particles are separated from the drying air in a cyclone, bag filter or a combination of both.
Depending on the product, either single or multi-stage drying is used. In single-stage drying, the final product moisture content is achieved within the drying chamber. In multi-stage drying, further water removal and cooling take place in fluid beds.
Powder separation/fines recovery
The air from the drying chamber and fluid bed(s) is discharged through exhaust ducts and the powder present in the exhaust air is separated in cyclones, bag filter(s) or a combination of both. The separated powder is collected at the bottom of the cyclone and/or bag filter and deposited fines are returned to the systems conveying line via a rotary valve located beneath the cyclone or bag filter. Depending on the desired product’s functional properties, the powder can be returned either to the fluid bed or the drying chamber.
Post-treatment of powder
The powder from the chamber undergoes further drying and cooling in a fluidised bed system, tailored to the specific type of product.
Fluidised beds are utilised for both single and multi-stage drying of agglomerated and non-agglomerated products. These beds facilitate gentle after-drying and/or cooling of delicate products. The well-mixed bed, whether static or shaking, ensures superior particle mixing, resulting in a uniform temperature and moisture profile throughout the powder, consistent with the outlet product flow. This homogeneity allows for high air velocity and turbulence, rapidly dispersing the feedstock in the fluid bed and preventing lump formation during this critical stage of the process. Additional drying and cooling are achieved in the plug flow sections following the well-mixed or static bed, providing accurate control over moisture and temperature levels. The vibrating or shaking motion of the fluid bed enhances air/solids contact, leading to improved product mixing and higher thermal efficiency. The drying and cooling air for the static or shaking beds is supplied by air handling units (AHUs).
Powder can also be cooled in a so-called Open Transport System. This system cools the powder while it is conveyed for a defined period in a duct to a cyclone in which the powder is then separated from the cooling air.
Agglomeration
Agglomeration practice
The term agglomeration typically refers to the formation of larger particles from smaller ones, which is essential for easy reconstitution in water. Specific processes that fall under agglomeration include tabletting, pelletising, extrusion, prilling, granulation, and sintering. The primary basis for agglomeration is particle enlargement through collisions between wet particles and spray droplets with dry particles. Common to these mechanisms is the formation of liquid bridges between particles or viscous layers around them. These types of agglomeration are performed in spray dryers, rewet agglomerators, and fluid bed granulators.
Benefits of agglomeration:
- Agglomeration is an essential step in making instant products. Non-agglomerated powders generally have poor wettability. This property severely limits their use in normal household conditions. Agglomeration changes the porosity of the powders, accelerating the penetration of water into the granules.
- Agglomeration is applied to improve the flowability and make powders less dusty or even dust-free. Such products are better to handle in further processing.
- Agglomeration is often an essential step prior to tabletting. It makes the product free-flowing and prevents segregation of components in cases where the final product is a mixture of various ingredients.
- Agglomeration changes the appearance of products and so can make products more attractive. From a marketing point of view, this might be an important issue.
- Agglomeration changes the bulk density of the product. This can also be important from a marketing point of view, for instance, to make the headspace in containers smaller.
Agglomeration in the drying chamber
During the spray drying process, the goal is to produce small particles. However, powders consisting of small particles are difficult to reconstitute and require intensive mixing to disperse the powder before it completely dissolves. Larger particles disperse more easily. Through agglomeration, both good dispersion and complete dissolution can be achieved.
Often, the base material for spray drying is a liquid with a high solid content. Wet spray droplets can also be used for agglomeration, a process known as straight-through agglomeration, which is conducted in spray dryers. The primary objective of agglomeration is typically to create instant properties.
To manufacture such products, agglomeration must be uniform and controlled to ensure the product is easy to disperse.
Straight-through agglomeration typically occurs in the atomisation zone, where the spray droplets are still wet and capable of coalescing. In practice, not all atomised particles undergo agglomeration; these non-agglomerated particles, known as fines, exit the dryer with the outlet air. After being separated from the air in cyclones and/or bag filters, the fines are collected and recycled back to the atomisation zone to agglomerate with the droplets.
Agglomeration is therefore a combination of:
- Collisions between moist spray droplets
- Collisions of dry, fine particles with moist spray droplets
Collisions between spray droplets can occur through the intersection of individual sprays, known as inter-spray agglomeration. Within individual spray jets, collisions between particles also occur, referred to as inner-spray agglomeration. The recirculation of fine dry particles to the atomisation area is crucial for the agglomeration process, as in many cases without recycling fines, the resulting agglomerates are often too small. Therefore, a successful straight-through agglomeration process combines inter-spray agglomeration and fines recirculation.
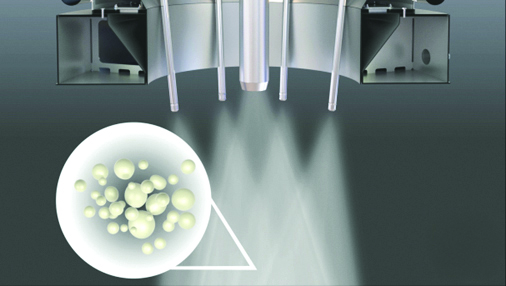
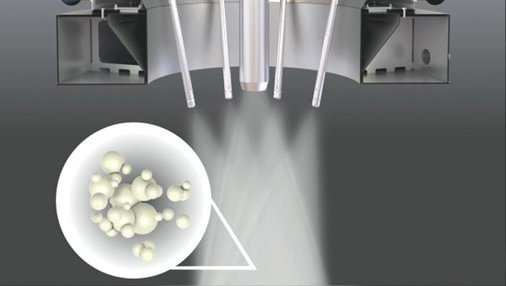
In most cases, the agglomeration process is performed with multiple spray nozzles where the individual sprays intersect. In the intersection or overlap zone, contact is made with fine and dry particles from the fines return. The spray lances can be adjusted inwards and outwards which allows for varying the distance of the drying trajectory to the intersection area. The length of this drying trajectory determines the humidity of the spray droplets in the overlap area, thereby controlling the nature of the agglomeration. The closer the lance tips are positioned to one another, and the fines return, the more agglomeration takes place. Figure 19.7 illustrates the degree of agglomeration of atomised concentrate and fines.
Fluid bed agglomeration
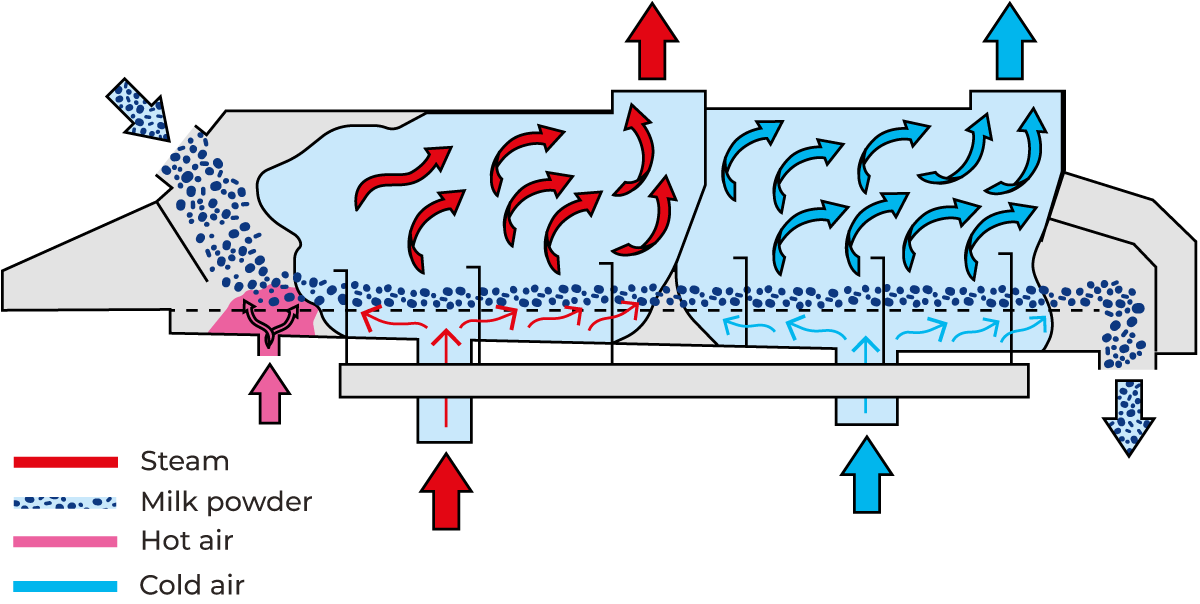
To achieve the desired porosity, the particles must first be dried so that most of the water in the capillaries and pores is replaced by air. Next, the particles are humidified to quickly swell their surfaces, closing the capillaries. This process makes the particle surfaces sticky, causing them to adhere and form agglomerates. One effective method of instantiation is re-humidifying agglomeration in a fluid bed, as illustrated in Figure 19.8. The fluid bed, connected to the discharge opening of the drying chamber, consists of a casing with a perforated bottom. Air at an appropriate temperature is blown through this perforated sheet at a velocity sufficient to suspend and fluidise the powder.
The housing is divided by means of a fine hole plate, separating the area into a lower air plenum and upper powder plenum, through which air is blown to fluidise the product. The fluid bed is mounted on springs and can be vibrated using a motor. The vibrating motion supports the fluidisation and conveying of the powder. Weirs, located between individual sections and at the outlet, regulate the height of the fluidised powder layer, while the length of the fluidised bed determines the duration of residence. Powder is transferred from the spray tower into the first section where it is humidified with steam. Airflow combined with vibrations, conveys the powder through successive drying sections, where hot air at decreasing temperatures is passed through the powder bed. Agglomeration takes place in the first stage of drying when the moistened particles adhere to each other to form agglomerates. The water is evaporated from the agglomerates during their passage through the drying sections. The powder is subsequently cooled and exits the fluid bed with the desired residual moisture and temperature. Large grains and fine grains are screened. The screened and instantised powder is then conveyed to filling by a gentle transport system. The outgoing air from the fluid bed, containing a certain amount of fine powder, is blown to the cyclone or bag filter of the main air system.
Single-stage drying
The choice between single-stage and multi-stage spray drying methods depends on various factors such as product characteristics, production requirements, and economic considerations.
In single-stage spray drying, the entire drying process, from liquid feed to dried powder, is completed in a single step within the drying chamber only. It is suitable for products where the desired moisture content can be achieved in a single drying step. Single-stage is simpler in design and operation compared to multi-stage systems and it is typically more cost-effective for moderate production volumes, and products with less stringent quality requirements. However, it may not be suitable for products that are sensitive to high temperatures like certain thermoplastic and hygroscopic products or products that require precise control over moisture content and particle properties. Figure 19.9 shows the arrangement of a single-stage spray drying plant.
Depending on the product, the incoming air is heated to a temperature of 150 – 250 °C. The heated air flows through a distributor which ensures that the air travels at a uniform speed into the drying chamber, where it is mixed with the atomised product.
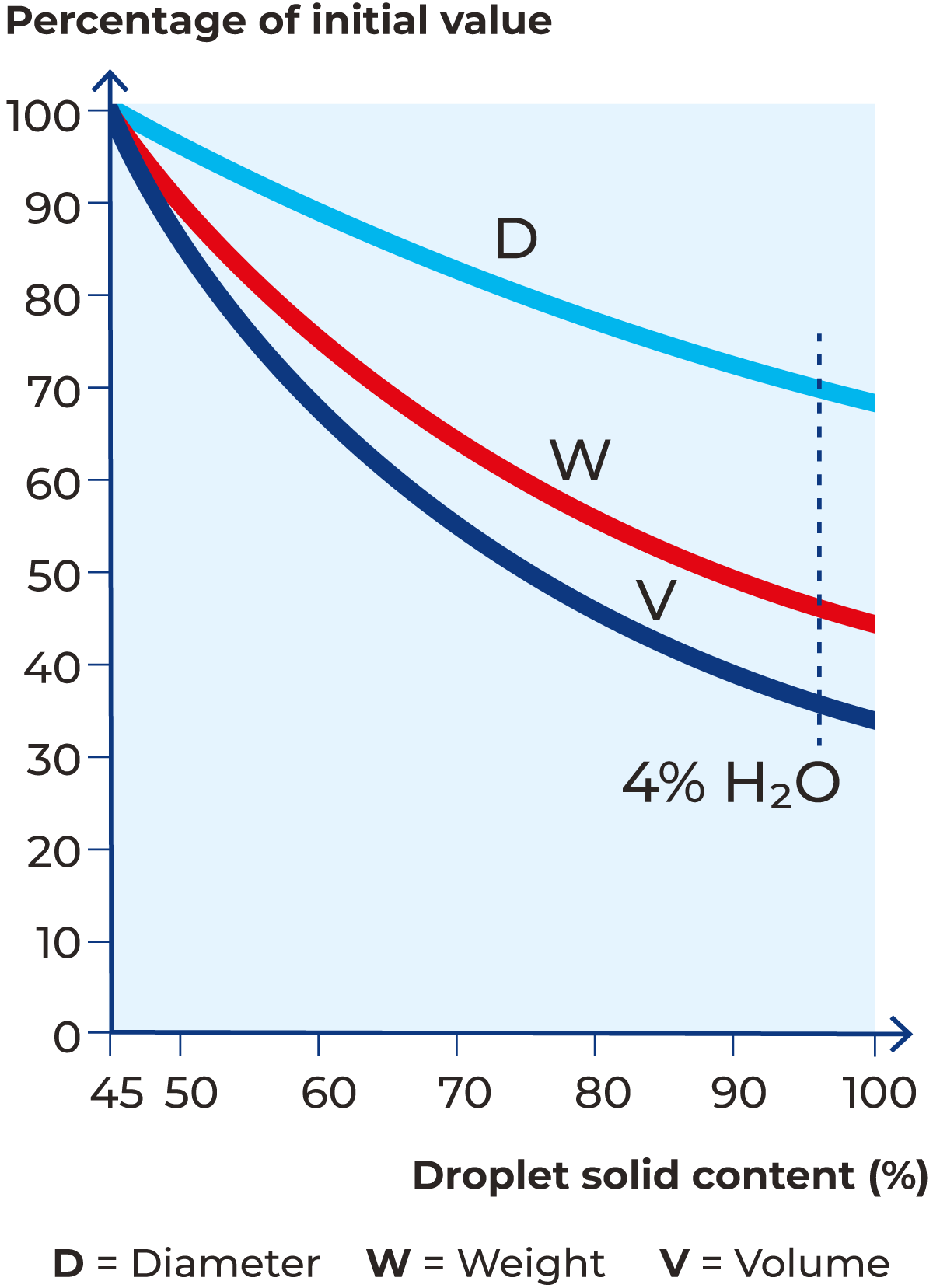
The free water evaporates instantly when the atomised product encounters the hot air that is blown into the drying chamber. Surface water evaporates quickly, as does the moisture from the inside of the droplets which quickly reach the surface by capillary action. Heat is transferred into the particles by convection which results in the evaporation of bound water, diffusing it onto the surface of the particles. As the heat content of the hot air is continuously consumed by evaporation of the water, the product heats up to a maximum temperature of only 15 – 20 °C below the temperature of the air when it exits the drying chamber; under normal conditions 75 – 95 °C. The evaporation of the water from the droplets leads to a considerable reduction in weight, volume, and diameter. Under ideal drying conditions, the weight will decrease by about 50% and the volume by about 40%. The diameter reduces to 75% of the droplet size after leaving the atomiser. Figure 19.10 depicts the reduction of weight, volume and diameter.
Multi-stage drying
The constant demand for improved product quality (flowability, dispersibility, lower dust content) improved product handling, better thermal and operational efficiency and environmental sustainability has instigated the need for further treatment of powder after discharge from the drying chamber. This has prompted the development of two and three-stage drying systems (multi-stage concepts), which are depicted in Fig. 19.11.
To enable multi-stage drying, the one-stage drying system is extended by means of one or more fluid bed dryers. The attached fluid bed can be a static/ well-mix type bed or a vibrating/shaking type (plug-flow) bed or a combination of the two, the latter often called three-stage drying.
The powder leaves the drying chamber with higher residual moisture and is further dried in the fluid bed(s) in which drying ends at relatively low temperatures and the powder may also be cooled. In terms of energy, this installation is better than a single-stage dryer and enables it to work with considerably lower air outlet temperatures. The quality of the powder can be improved by the separation of the fine powder in the fluid bed.
The static bed design is suitable for products that are directly fluidisable on leaving the drying chamber. The vibrating or shaking bed design is suitable for products that are more difficult to fluidise due to their wide particle distribution, fine particle size and irregular shape.
Two-stage drying in the fluid bed dryer ensures that the desired residual moisture is achieved and that the powder is cooled. Final drying in the integrated fluid bed dryer ensures that the desired residual moisture is achieved. The installations can be operated with both nozzle and centrifugal atomisers.
Although the advantages of multi-stage drying are obvious, one of the challenges can be the drying conditions where product starts adhering to equipment surfaces. In this situation, an integrated static bed may offer a viable solution since it can handle elevated moisture levels better.
The choice between single-stage and multi-stage drying depends on factors such as the product properties, desired moisture content, required throughput and energy efficiency considerations. Single-stage drying is simpler and more economical for straightforward drying tasks, while multi-stage drying offers enhanced control and flexibility, making it suitable for more challenging drying applications. Multi-stage drying can be applied to a wide range of dairy products such as agglomerated and non-agglomerated whole and skim milk, whey concentrate, whey and milk protein, whey permeate, caseinates, baby food, etc.
Energy consumption single vs multi-stage drying
In single-stage spray drying, the liquid feed is atomised into a hot drying chamber where all the water is evaporated in one step. Single-stage drying generally requires more energy because the entire drying process relies on the hot air introduced in one stage to evaporate all the moisture. The exhaust air still contains significant amounts of heat that is not reused.
Advantages:
- Simpler design and operation
- Lower initial capital costs
Disadvantages:
- Higher operational energy costs due to less efficient use of heat
- Higher thermal stress on the product, which can affect quality
Multi-stage spray drying involves more than one drying stage. Typically, the initial stage is a standard spray drying process, followed by additional stages such as fluid bed drying. The additional stages (like integrated fluid beds or external fluid beds) help to further dry the product using lower-temperature air.
Multi-stage drying systems are more energy efficient. After the initial high-temperature drying stage, subsequent stages use lower-temperature air to complete the drying process.
Advantages:
- More energy-efficient due to the use of lower temperature air in subsequent drying stages and heat recovery
- Better product quality due to gentler drying conditions in later stages
- Lower operational costs over time due to reduced energy consumption
Disadvantages:
- More complex design and operation
- Higher initial capital costs due to the additional equipment and stages
Multi-stage spray drying systems can save about 10% of energy compared to single-stage systems due to the more efficient use of drying stages. Two-stage drying involves spray drying to a moisture content which is about 4% higher than the required final moisture content. The residual moisture is then removed from the powder with fluid bed drying. With multi-stage drying the outlet air temperature is approximately 15 – 20 °C lower than with a single-stage which also reduces the thermal load on the powder particles leaving the drying chamber. Multi-stage drying also allows a higher inlet air temperature which in turn contributes to improved thermal efficiency and hence improved economics. The specific savings can vary based on the system design and operational conditions, but these percentages provide a general idea of the potential energy efficiency improvements.
For both single and multi-stage drying systems, heat recovery can be applied, allowing the reuse of surplus heat in the exhaust air, which can reduce the overall energy consumption by 10 – 15%.
Spray dryer main equipment
The main components of a spray dryer typically include:
- Drying chamber
- Air supply system
- Air distribution system
- Feed system
- Atomisation system
- Powder recovery system
- Fines return system
- Fluid bed drying and cooling system
- Lecithin system
- Fire and explosion protection system
- CIP system
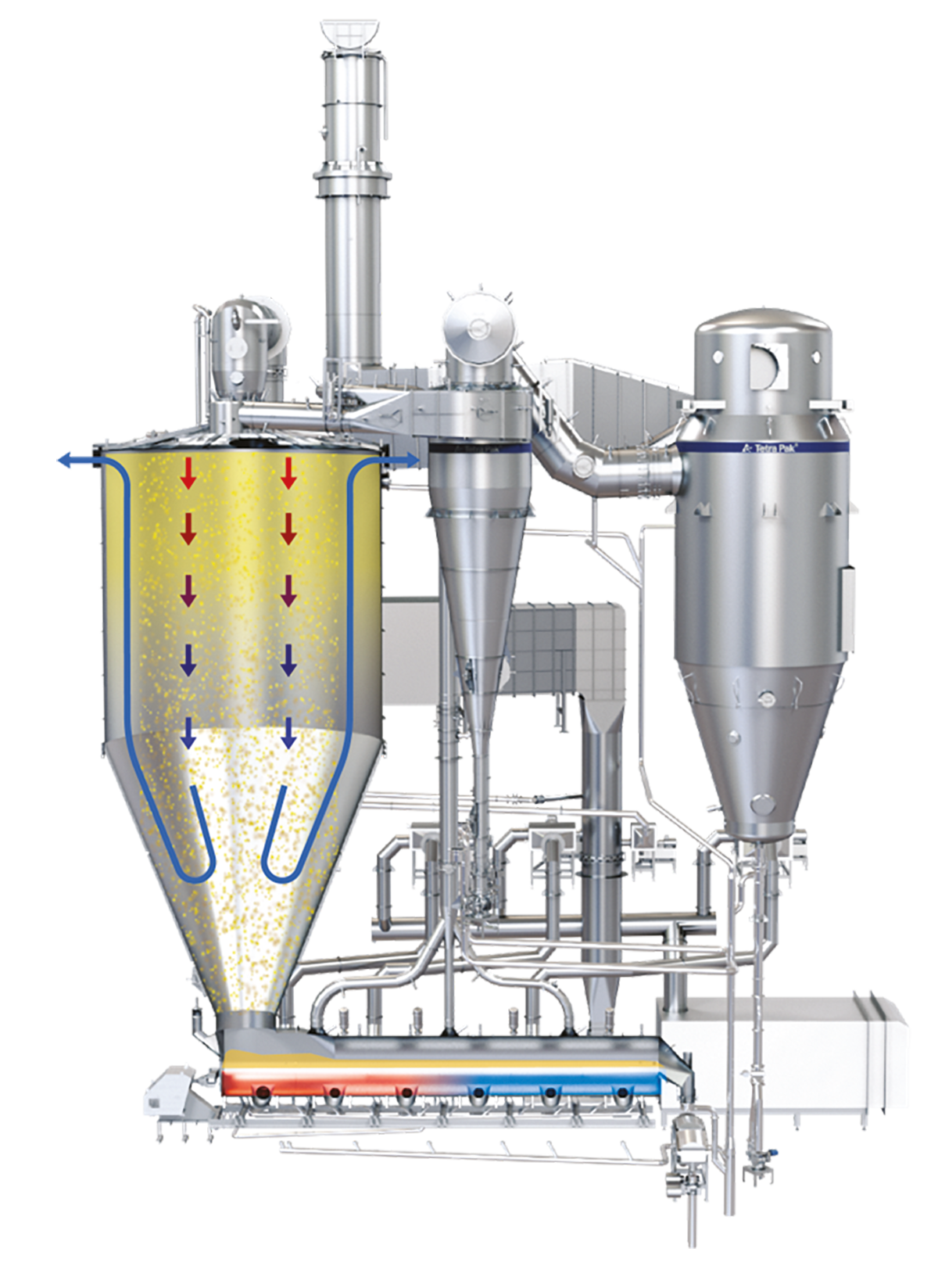
Drying chamber
The industry offers a wide range of drying chamber designs. The most common one seen for dairy powder is the cylindrical chamber with a cone section. A cone angle ranging between 40 to 50 degrees facilitates the smooth discharge of powder at the bottom outlet of the chamber.
The two types of drying chambers that are mostly used are:
- Wide Body dryer
- Tall Form Bustle dryer
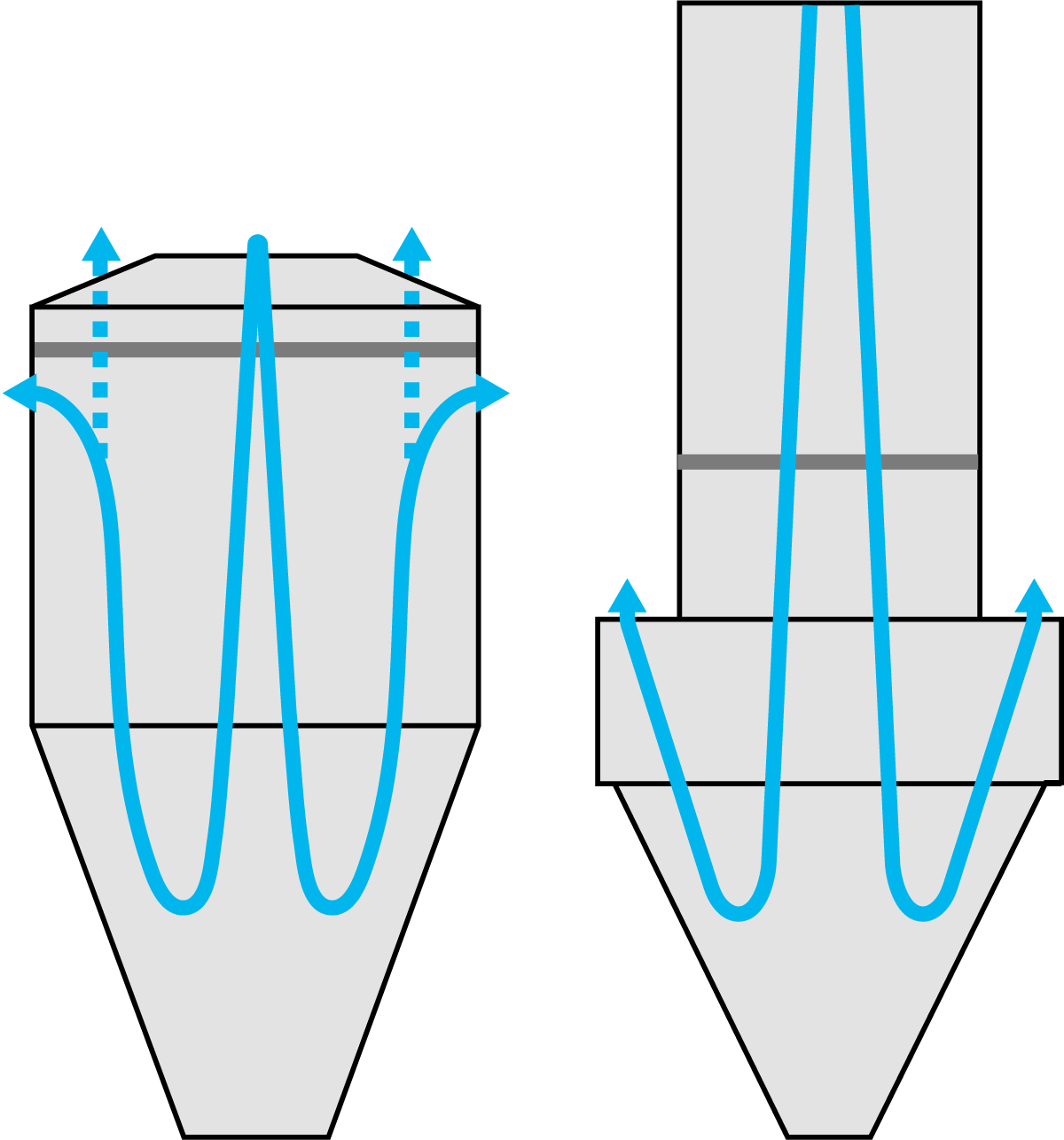
The Wide Body concept relies on a co-current, reversed airflow pattern in the drying chamber, creating a barrier of fines containing relatively cold process air between the hot air jet in the centre and the dryer wall, thus minimising the formation of powder deposits on the chamber wall. The process air discharges at the top of the cylinder, either at the sides or from the top of the chamber roof. The air in the cylindrical section travels plug flow and reverses in the conical section. Due to the reversing of the airflow, the coarse particles are separated from the air by gravity and discharged at the bottom outlet of the dryer cone. The smaller particles (fines) are entrained in the air stream and leave the drying chamber at the air outlets. See Figure 19.12.
The Tall Form Bustle concept features air discharge at the top of the conical section, at the bustle. Here, the airflow follows a plug flow pattern in the cylindrical section and reverses within the conical section. Like the Wide Body design, this reversal of airflow causes coarser particles to separate from the air by gravity which then discharge at the bottom of the dryer cone. The smaller particles (fines) are entrained in the air stream and leave the drying chamber at the bustle. See Figure 19.12.
The selection of the dryer type is subject to the type of product to be spray dried. The Wide Body dryer is suitable for a wide range of products whereas the Tall Form Bustle dryer is typically used for low or non-fat products like whey, permeate, and whey proteins.
The drying chamber is equipped with automatic operating hammers to dislodge any powder that may have accumulated on the chamber wall.
The (under) pressure in the drying chamber is controlled by a pressure indicator controller (PIC), controlling the speed of the main exhaust fan(s). To protect the drying chamber from too low pressure, a pressure switch is installed. In case the vacuum in the drying chamber exceeds a certain set point, an emergency stop is initiated, and the main inlet and exhaust fans are stopped.
Air supply system
The air supply system typically includes the following equipment:
- Louvres
- Noise attenuator
- Pre and final filters
- Dehumidifier
- Pre-heaters
- Main air heater
- Air supply fan
- Air distributor
Ambient air required for the drying process passes through an arrangement of components including a louver, winter coil, set of filters and a silencer before it is distributed to the main and secondary air process systems.
The main air supply fan blows filtered air, required for the main drying process through the main air heater and the air distributor into the drying chamber.
The primary drying air can be heated by the following heating systems:
- Steam heater
- Indirect gas heater
- Thermal oil heater
- Electrical heater
The drying air is heated to a temperature between 150 and 250 °C, depending on the available heating medium and type of product to be dried and is blown into the dryer chamber through the air distributor which ensures an optimal distribution of drying air into the drying chamber.
Depending on the ambient conditions, the water content of the drying air may reach a point where it can’t absorb additional moisture effectively. In such cases, dehumidification becomes necessary. This is achieved by cooling the air below its dew point using chilled water. The condensed water is then separated from the airflow using a so-called demister. Subsequently, the air is reheated to the desired drying air temperature.
A more advanced technique to remove water from the air is dehumidification by means of adsorption of moisture with silica gel or another type of desiccant.
Feed system
The dryer feed system typically comprises the following components:
- Feed tank
- Concentrate feed pump
- Pre-heating system
- Filter
- High-pressure pump or rotary atomiser
- High-pressure feed line with high-pressure valves and lances
Concentrate from the evaporator, crystallisers or other concentrate storage is collected in the dryer feed tank to ensure constant total solids to the spray dryer. The feed tank can be single or double-executed depending on the type of product and product temperature. A multiple-tank system is typically used in the case of products with sensitivity to microbiological growth in a certain temperature range. In such a situation, one concentrate tank is in use at any given time while the other undergoes CIP (cleaning in place) or remains on standby. The concentrate from these tanks is pumped to one or more high-pressure pumps or rotary atomisers using either centrifugal or positive displacement pumps. The tanks are usually switched every 4 to 10 hours.
Depending on the type of product, the concentrate can be heated in a tubular or plate heat exchanger from 10 °C up to 80 °C to adjust viscosity prior to atomisation. After heating, the concentrate is filtered to remove any objectionable or foreign matter.
The high-pressure pump provides the pressure to atomise the liquid feed into very fine droplets for effective drying. Depending on the type of product and capacity, multiple nozzles are deployed. Each product has its own so-called specific lance load expressed in litre or kilogram per hour per lance/nozzle.
The high-pressure pump can be equipped with homogenizing valves to create a uniform and stable mixture. The core principle behind a homogenizer is to force the mixture through a narrow space under high pressure. This high shear force breaks down the particles or droplets, resulting in a homogeneous mixture. One example is the homogenization of whole milk to reduce the free fat content in the powder.
Atomisation system
The most used atomisation systems are:
- High-pressure nozzle atomiser
- Rotary disc atomiser
- Two-fluid atomiser
Pre-heated concentrate can be either atomised by means of an arrangement of high-pressure nozzles, a rotary disc atomiser or two-fluid nozzles.
The basic function of pressure nozzles is to convert the pressure energy supplied by the high-pressure pump into kinetic energy to form a spray of fine droplets. The high-pressure line feeds the high-pressure nozzle system which consists of multiple high-pressure valves that supply concentrate to the lances located in the dryer air distributor.
In rotary disc atomisers, the liquid is continuously accelerated to the wheel circumference by centrifugal force, produced by the rotation of the wheel. The liquid is distributed centrally and extends over the wheel surface in a thin sheet, discharged at high speed at the periphery of the wheel. When the thin film of liquid reaches the periphery, the centrifugal force causes the liquid to break up into fine droplets, creating a spray of atomised particles. The characteristics of the spray such as droplet size and distribution, depend on several factors, including the rotational speed of the disc, the properties of the liquid (such as viscosity and surface tension), and the design of the disc (such as diameter and surface texture).
In two-fluid or pneumatic atomisers, the feed is atomised by means of high-velocity air for which compressed air is used. Two fluid atomisers are typically used for small-scale (pilot) dryers or other applications for which small quantities of feed must be atomised into a fine spray. One example is the spraying of lecithin onto certain types of powder to obtain instant properties.
Powder recovery system
The powder recovery system typically consists of:
- Cyclone
- Bag filter
A classic system for the separation of powder from drying air is a cyclone or arrangement of cyclones in series or in parallel. The residual fines content in the outgoing air after the cyclone(s) is relatively high, 100 – 200 mg/Nm3. Introducing bag filters allows the residual fines content to be lowered to less than 10 mg/Nm3, which is generally required by local authorities for environmental purposes.
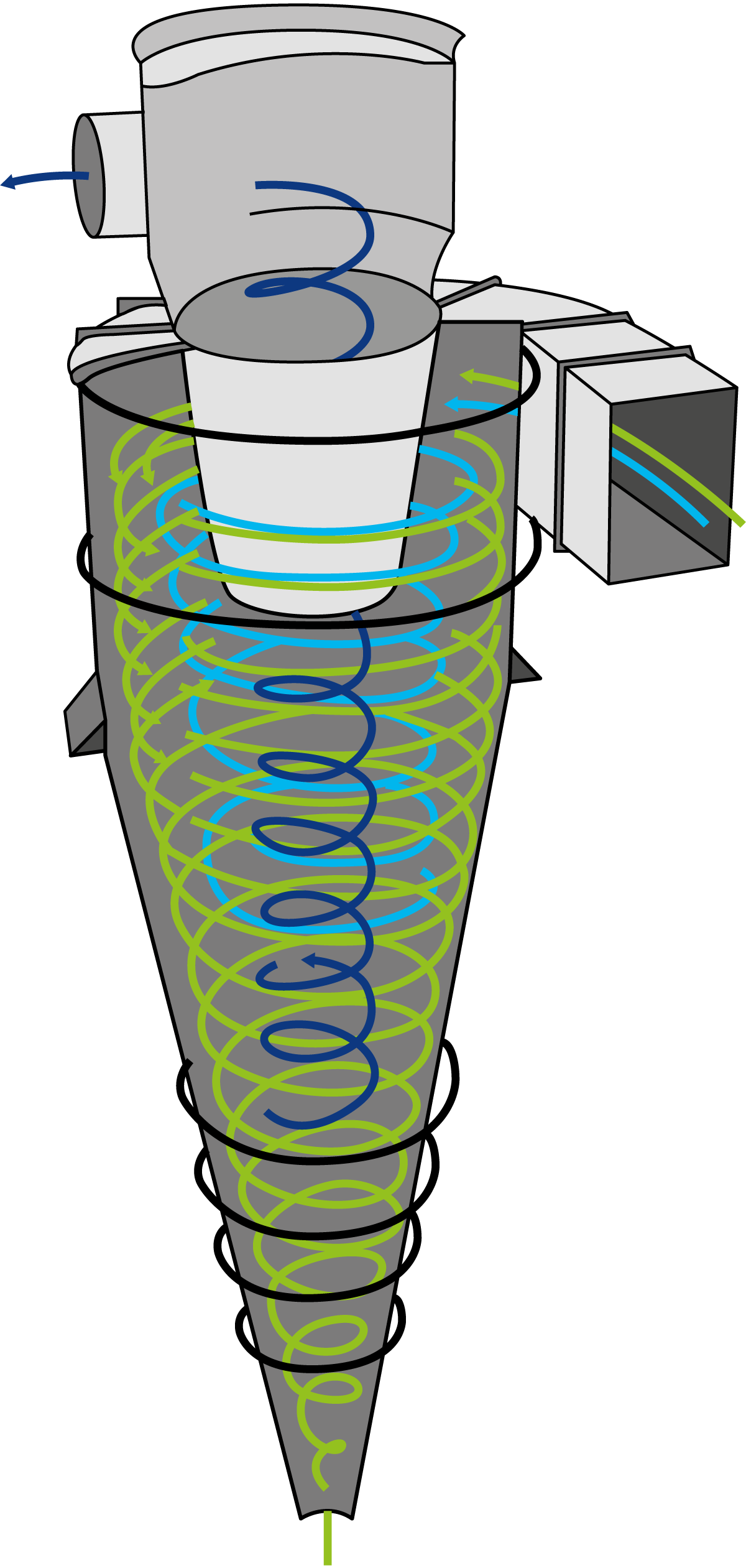
The working principle of cyclones involves the use of centrifugal force to separate particles from a gas or liquid stream. The mixture of air and powder particles enters the cyclone through a tangential inlet at high velocity. This design causes the stream to spiral downward along the inner walls of the cyclone. As the stream spirals downward in a circular or helical path, the centrifugal force pushes the heavier particles outward toward the cyclone’s walls. The lighter gas or liquid remains closer to the centre of the cyclone. The particles, now concentrated near the walls, lose their momentum due to friction and gravity. They begin to slide down the walls to the bottom of the cyclone, where they are collected in a collection chamber or chute. The cleaned air, now mostly free of particles, moves toward the centre of the cyclone and is drawn upward through a central vortex finder (an inner tube) to the outlet at the top of the cyclone. In the cyclone, two vortices are formed: an outer downward vortex carrying the particulate-laden gas or liquid, and an inner upward vortex carrying the cleaned gas or liquid. These vortices are crucial for the separation process.
Bag filters are used to ensure maximum recovery of product and to meet local dust emission requirements. The powder present in the exhaust air will be separated in a CIP-able bag filter. The fines containing air from the drying chamber or cyclones enter the bag filter radial at the “bags level”. A baffle plate in the filter provides an even distribution of air to the filter bags. Inside the baghouse, the air passes through multiple filter bags made from food-grade material, often polyester. The filter bags are usually cylindrical and are arranged in ring-shaped rows.
As the air passes through the filter bags, the particulates are trapped on the surface or within the pores of the filter material. The cleaned air then exits through the top of the bags and is released through the air exhaust. Over time, dust and particulate matter accumulate on the surface of the filter bags, forming a dust cake. This dust cake helps in filtering finer particles as it adds to the overall filtration efficiency. To maintain the efficiency of the bag filter, the filter bags must be periodically cleaned to remove the accumulated dust. Pulse-jet cleaning is the most common and efficient method to clean the filter bags. A short burst of compressed air is directed into the bags, causing them to expand and release the dust cake. The dislodged fines are collected in the fluidising bottom cone of the filter house and discharged through a rotary. After passing through the filter bags and being cleaned of particulates, the air is discharged through the exhaust fan into the atmosphere.
The residual fines accumulated in the bottom of the bag filter or cyclones can either be returned to the process or treated as waste as far as baby food and baby food ingredients are concerned.
During plant shutdown, the electrically heated air handling unit is used as weekend heating. The cone, product side, and clean air side are equipped with access doors. The bags can easily be replaced via the large access door on the clean air side.
The use of washable filters (CIP-able), which are linked to the CIP system, is currently state of the art. Regular cleaning ensures that the residual fines are not contaminated and remain suitable as food, which leads to an increase in yield and thus a reduction in powder manufacturing costs. Residual fines from baby food or baby food ingredients are typically not recovered for food safety reasons as the product may contain fibres that release from the filter bags during pulsing.
Fines return system
The fines return system comprises the following equipment:
- Blower
- Blow through rotary valves
- Conveying line
- Divert valves
- Fines return pipe in air distributor
The powder from the cyclones and/or bag filter is recovered and discharged through rotary valves and subsequently conveyed by means of a middle-pressure transport system to any of the following destinations.
- The atomisation zone to agglomerate with the droplets
- Front of the fluidising bed for regular, non-agglomerated products
- End of the fluidising bed to empty out the system at the end of production
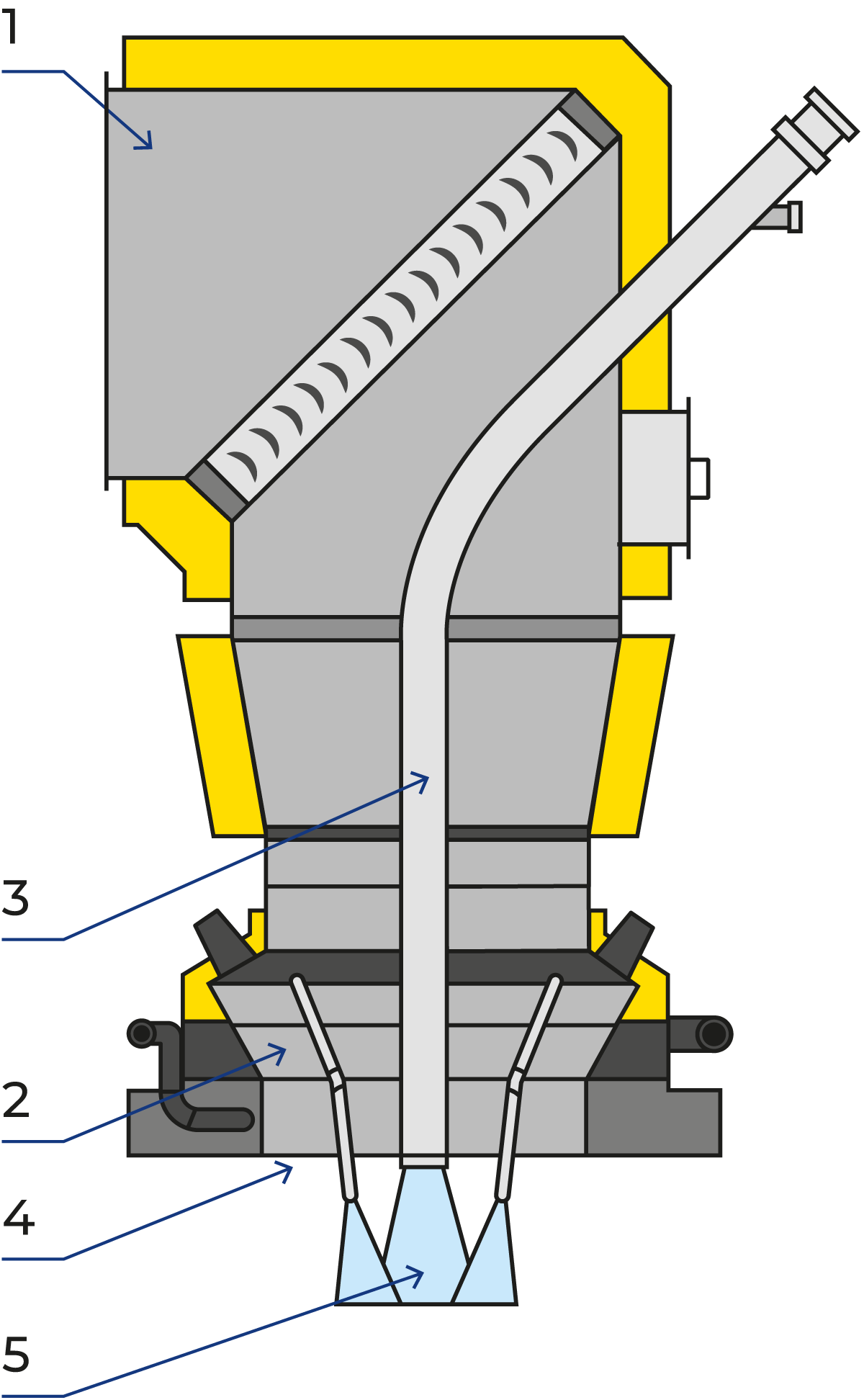
To achieve good agglomeration, the fines must be introduced into the atomising zone to ensure intimate contact with the droplets and fines. A fines-return tube with a cooling jacket located inside the air distributor ensures that the recovered powder from the cyclones and/or bag filter is introduced into the atomisation zone effectively. Figure 19.13 shows the fines return pipe surrounded by the high-pressure nozzles.
Fluid bed drying and cooling
The fluid bed drying and cooling system typically includes the following equipment:
- Integrated static bed
- Vibrating or shaking fluid bed
- Air handling unit (filter, fan, heater, cooler, demister)
Powder from the chamber is further dried and cooled in a fluid bed arrangement. The system used depends on the type of product. Fluid beds are used for single and multi-stage drying and allow gentle after-drying and cooling of agglomerated and non-agglomerated products.
Integrated static fluid beds consist of a cylindrical vessel mounted underneath the drying chamber at the cone outlet. The static bed is divided into the lower air plenum section and the upper powder section. The lower and upper sections are separated by a perforated plate or mesh through which heated or cooled/conditioned air is blown, creating an upward flow that lifts and separates the particles.
Vibrating or shaking fluid beds typically consist of an elongated horizontal vessel divided into a lower air plenum section and an upper powder plenum section. Like the static bed, the lower and upper sections are separated by a perforated plate or mesh, through which heated or cooled/conditioned air is blown, creating an upward flow that lifts and separates the particles.
The milk powder particles become suspended in the air stream, forming a fluid-like state. This ensures that each particle is surrounded by air, promoting uniform drying and cooling. As the particles are fluidised, they are exposed to conditioned air which can be heated for drying or cooled for cooling. This helps in removing any residual moisture and bringing the powder to the desired temperature. The dried and cooled powder is then discharged from the fluid bed for further processing or packaging.
To achieve the desired drying or cooling effect, a relatively high quantity of air must be used. The air passes through the perforations at high velocity to prevent powder particles from falling through the perforated plate. However, the average air velocity above the plate, known as the fluidising velocity, must be considerably lower to avoid blowing off the powder from the fluidising bed into the exhaust.
Depending on the type of product and type of fluid bed, the fluidising velocity ranges from 0.2 – 1.2 m/s. High-fat/agglomerated products like whole milk powder generally allow higher fluidising velocities than low-fat/non-agglomerated products like skim milk powder. Static beds typically require higher fluidising velocities due to the amount and high level of powder, and the absence of a vibration or shaking motion.
The drying and cooling air for the static or shaking beds is supplied by means of air handling units. The AHUs for the drying section consist of a fan, static filter(s) and a steam air heater for the required air temperature.
The AHUs for the cooling/conditioning sections consist of a fan, static filter(s), an air cooler, and a steam air heater. The air cooler provides conditioned air with low relative humidity, preventing the powder from absorbing moisture from the air during cooling.
Lecithin system
The lecithin system comprises:
- Lecithin tank(s) with agitator and heating jacket
- Heat traced lecithin recirculation loop and supply
- Heat-traced compressed air supply
- Lecithin lance with two-fluid nozzle
Lecithination involves the coating of powders with a wetting agent which usually is lecithin dissolved in butter oil or another type of oil like vegetable oil. The lecithin concentration in the fat can be as high as 60% and the mix is added to achieve a lecithin content of 0.2 – 0.5% in the powder. The lecithin/oil mix is sprayed using a two-fluid nozzle directly onto or into the powder during fluidisation in a static or vibrating/shaking fluid bed.
To ensure effective mixing of lecithin with powder, the temperature of the lecithin/oil mix shall be 50 – 60 °C, while the compressed air to atomise the lecithin mix should have at least the same temperature or higher (60 – 70 °C). The final powder temperature after lecithination and cooling shall not be too low to ensure the required functional properties, like wettability, are met.
Powder sifter
After the powder has been dried and cooled to the final moisture content and temperature, the powder is sieved to separate oversized particles like lumps, leftover agglomerates, and other objectionable matter. The sifter usually consists of an enclosure with a mesh inside to separate oversized particles. The sifter can have either a vibrating or shaking motion to facilitate transport and separation.
Fire and explosion protection system
The fire and explosion protection system typically consists of:
- Fire extinguishing system
- Explosion vent panels
- Explosion suppression and isolation system
- Fire and explosion control system
The powder mixture in the drying chamber is often combustible, which may lead to fires resulting from smouldering spots like embers. Fires can be extinguished by installing extinguishing nozzles in the chamber roof, the fluid beds and the bag filter. Carbon oxide (CO) detectors or photoelectric cells are used to detect smoulder spots at an early stage, far before they can initiate a fire or explosion.
Destruction and thus long-term loss of plant function can be avoided by installing pressure relief panels or rupture discs which keep the pressure on the wall of the chamber low in the event of explosions. Ducts and filters can be protected by extinguishing barriers which are triggered by means of pressure detectors or infrared sensors. These include fire extinguishers or suppression bottles pre-filled to about 50 bar, mounted permanently at strategically important locations and filled with inert powder (sodium bicarbonate). In the event of an explosion, a detonator blows up the partition disc between the extinguishing container and the dryer. The extinguishing powder is blown into the plant instantaneously and brings the area where it was blown into a non-flammable state by changing the powder-to-air ratio. The explosion front thereby stops it from spreading.
The nozzles and sprays are often monitored using a camera system that can detect abnormalities like inconsistent sprays or leaking nozzles in an early stage before they initiate a fire and explosion.
Spray-dried milk products
Application of milk powders
Dried milk can be used for various applications such as:
- Recombination of milk and milk products
- In the bakery industry to increase the volume of bread and improve its water-binding capacity. The bread will then remain fresh for a longer period.
- Substitute for eggs in bread and pastries
- Producing milk chocolate in the chocolate industry
- Producing sausages and various types of ready-cooked meals in the food industry and catering trade
- In baby foods
- Production of ice cream
- Animal feed, calf growth accelerator
Dried milk powder is a dehydrated dairy product manufactured by evaporating milk into a dry material. Natural milk consists of four main constituents: protein, fat, lactose, and minerals. It is common practice to standardize the fat and protein content of milk to global standards. Proteins are standardized by adding lactose or milk permeate. The fat content is adjusted by centrifugation or the addition of cream to achieve the fat content required in the final product.
One purpose of drying milk is to preserve it; milk powder has a far longer shelf life than liquid milk and does not need to be refrigerated, due to its low moisture content. Another purpose is to reduce its bulk for economy of transportation. Powdered milk and dairy products include such items as dry whole milk, non-fat dry milk, dry buttermilk, dry whey products, whey, and milk derivatives like MPC and WPC and dry dairy blends. Many dairy products exported conform to standards laid out in Codex Alimentarius.
Each field of application makes its own specific demands of milk powder. If the powder is to be mixed with water for direct consumption (recombination), it must be readily soluble and have the correct taste and nutritive value. For this application, the product must be dried very carefully in a spray dryer. Some degree of caramelisation of the lactose is beneficial in chocolate production. Here, the powder can be subjected to intensive heat treatment, for instance in a roller dryer.
Milk powders may be categorised as follows:
- Dairy: whole milk powder, skim milk powder, milk protein powder, casein/caseinate powder
- Whey: sweet whey powder, fat-filled whey powder, whey protein powder, whey permeate
- Nutritional: nutritional and infant powder
Whole milk powder (WMP)
Whole milk powder is usually obtained by removing water from pasteurized, homogenized whole milk. It may also be obtained by blending fluid, condensed or skimmed milk powder with liquid or dry cream, or with fluid, condensed or dry milk. After standardization of the fat content, the milk does not need to be homogenized if it is thoroughly agitated, without air inclusion. Homogenization is normally carried out between evaporation and spray drying. Whole milk powder must contain between 26% and 40% milk-fat (by weight) on an “as is’’ basis, a minimum of 34% total protein and not more than 5.0% moisture (by weight) on a solids-non-fat (SNF) basis.
Milk intended for whole milk powder is pasteurized at 75 °C for 15 seconds to destroy most of the pathogenic bacteria and to inactivate enzymes that would otherwise degrade the milk during storage.
Whole milk powders can be produced using various types of dryers like roller or spray dryers, the latter being most common. Whole milk powder is typically dried multi-stage (two or three stages) for economic reasons and to obtain the required powder functional properties. Whole milk powder is produced as regular non-agglomerated or agglomerated powder.
For regular whole milk powder, the bulk density is one of the most important parameters. Regular whole milk powder requires a high bulk density which can be achieved by avoiding agglomeration, thus returning the recovered fines from the cyclone to the fluid bed. There are, however, many other parameters that affect the bulk density, like the total solids (viscosity) of the dried concentrate, type of atomisation, dryer temperature profile, powder moisture, etc. Too moist powder in the fluid bed may, for instance, enhance agglomeration. The powder conveying line from the fluid bed to the powder silo may facilitate the breakdown of the agglomerate in favour of high bulk density.
For agglomerated whole milk powder the aim is to obtain excellent dissolving properties like – amongst others – wettability and dispersibility. Agglomerated whole milk powder is produced by returning the recovered fines from the cyclone or bag filter to the atomisation zone, where they will coalesce with the wet sprays to form agglomerates. In addition, the agglomerated powder is usually lecithinated in the fluid bed to enhance wetting-ability. Vitamins and minerals can also be added in the liquid feed for fortification prior to evaporation. Transport of the agglomerated powder from the fluid bed to the powder silos must be carried out gently to minimise breakdown. For this reason, dense phase conveying systems are often utilised.
Whole milk powder, regular or instantised, is typically dried multi-stage on a Wide Body spray dryer at an inlet temperature of 200 – 220 °C and an outlet temperature of 75 – 80 °C. The feed total solids range between 50 and 52%.
Application:
- Reconstituted milk
- Yoghurt and ice cream
- Bakery and confectionary
- Savoury products
- Nutritional and dietary products
- Infant formula
Non-fat dry milk and skim milk powder (NFDM/SMP)
Non-fat dry milk and skimmed milk powder are very similar. Both are obtained by removing water from pasteurized skimmed milk. NFDM and SMP contain 5% or less moisture (by weight) and 1.5% or less milk fat (by weight). The difference is that skimmed milk powder has a minimum milk protein content of 34% SNF (solids non-fat), whereas non-fat dry milk has no standardized protein level. The protein in skim milk is standardized with lactose or milk permeate. The production process of skim milk powder involves separation, standardization, pasteurization, heat treatment, evaporation and drying. Non-fat dry milk and skimmed milk powders are available in roller-dried and spray-dried forms, the latter being the most common. Spray-dried non-fat dry milk and skimmed milk powders are available in two forms: regular, non-agglomerated (non-instant) and agglomerated (instant).
Non-fat dry milk and skimmed milk powder are classified for use as ingredients in the food industry according to the heat treatment used in their manufacture. There are three main classifications: high-heat (least soluble), medium-heat, and low-heat (most soluble). They are classified into categories related to the temperature/time combinations to which the skim milk has been exposed prior to and during evaporation and drying. Heat treatment denatures whey proteins, with the percentage of denaturisation increasing with the intensity of the heat treatment. The degree of denaturation is normally expressed by the Whey Protein Nitrogen Index (WPNI), i.e. in milligrams of undenatured whey protein nitrogen per gram of powder. Information about the various categories of spray-dried skim milk powder is summarised in Table 19.4.
Skim milk powders, regular or instant, can be dried single or multi-stage on a Tall Form Bustle or Wide Body spray dryer at an inlet temperature of 200 – 230 °C and an outlet temperature of 80 – 85 °C. The feed total solids ranges between 48 and 51%, depending on the heat treatment. Higher heat treatment generally requires lower total solids due to the denaturation of proteins and hence increase in viscosity.
The main applications for regular skim milk powder are the bakery industry, the recombining industry, and the milk powder industry for fat-filled powder. Agglomerated skim milk is often used as coffee whitener which requires good instant properties and flowability.
Application:
- Reconstituted milk
- Yoghurt and ice cream
- Bakery and confectionary
- Savoury products
- Nutritional and dietary products
- Infant formula
Fat-filled milk powder (FFMP)
In fat-filled milk powder, the butterfat is often replaced by vegetable oil or other types of oil. The production process involves mixing skim milk concentrate with a fat blend at a temperature well above the melting point of the fat. The concentration of skim milk from the evaporator must be adjusted to achieve a final concentration of ~50% for spray drying after the fat is added. Fat blends usually contain emulsifiers and stabilisers as well as other additives such as vitamins and minerals.
One of the challenges for a spray dryer is the high fat content in powder. The higher the fat content the more difficult it is to dry the product. For fat content up to 35%, the standard dryer design for whole milk powder can be used without adjustments. Above this fat level, measures shall be taken to prevent powder deposits in the dryer. Measures may include lower inlet air temperature, lower air velocities, specially designed powder conveying lines, appropriate homogenization pressures, and lower atomisation pressure.
Fat-filled products are intended as ingredients in different food industries, primarily in baking, for calf feed mixtures, and whole milk replacers for human consumption.
Buttermilk powder (BMP)
Buttermilk is the liquid (serum) that remains after butter has been churned from cream. Due to the presence of naturally occurring lactic acid bacteria, traditional buttermilk has a tangy, slightly sour flavour. Traditional buttermilk is low in fat since most of the fat is removed with the butter. It is comparable with skim milk but contains about 5 to 10 times more milk fat. Buttermilk retains some of the proteins, vitamins, and minerals found in milk. Buttermilk can be spray dried using approximately the same drying conditions as skimming milk.
The main applications for buttermilk powder are for baking (pancakes, waffles, biscuits), cooking, beverages, snacks and confectionary, processed food, etc.
Milk protein powder
Milk protein powder is a concentrated form of milk protein commonly used in various food products and nutritional supplements. Milk protein powder is made by removing most of the water, lactose, fat and minerals, resulting in a high-protein powder.
Types of milk protein powder:
- Casein
- Caseinate
- Milk protein concentrate and isolate (MPC, MPI)
- Micellar casein concentrate and isolate (MCC, MCI)
- Whey protein concentrate and isolate (WPC, WPI)
Milk proteins include a complex of caseins (a-, β-, κ-caseins), and serum proteins like a-lactalbumin, β-lactoglobulin, and serum albumin. Casein makes up about 80% of the milk protein, while serum protein accounts for about 20%. These two groups have different chemical and physical properties. Casein is sensitive to acids and rennet enzymes, which causes it to coagulate into curd. Serum proteins are water-soluble and remain in the whey after casein precipitation. Casein digests slowly, providing a sustained release of amino acids over several hours, while serum protein digests rapidly, leading to a quick spike in amino acids after consumption.
In powdered form, milk protein MPC typically contains 70 – 90% protein, with a low fat and lactose content, especially in isolates, and vitamins and minerals such as calcium, potassium, and phosphorus.
Casein
Two types of casein exist: rennet casein and acid casein. Rennet casein is obtained through enzymatic precipitation, while acid casein is obtained by acidifying skim milk to its isoelectric point (pH 4.6 – 4.7). Both acid and rennet casein involve precipitation, washing, and concentration, followed by drying. The acid or rennet casein curd is typically dried in a fluid bed dryer.
Application:
- Processed cheese, and protein supplements for slow digestion
- Cookie formulations
- Nutritional and dietary products like sports and medical nutrition
- Cured meat and sausages
Caseinate
Caseinates are compounds derived from casein and can be subdivided into calcium-, sodium-, and potassium caseinate. Calcium-, and sodium caseinate are most common. Each of these caseinate types has its specific characteristics and application.
Caseinate concentrates are viscous solutions that limit the concentrate solids content depending on the type of caseinate. If caseinate solutions are too viscous, filamentation (the development of little threads) can occur upon atomisation. These filaments are coarse and adversely affect proper spray drying. The occurrence of filamentation can be avoided by using a specially designed two-fluid (steam) nozzles for atomisation. Traditionally, high-pressure nozzles are used for atomisation to obtain a high bulk density. The total solids content of the feed to the spray dryer is limited by the viscosity, and should not be more than 20 – 22%. However, higher total solids of up to 25% is possible when using two fluid (steam) atomisation. The selection of two-fluid atomisation is, nevertheless, subject to the cost of steam versus the cost savings resulting from higher total solids.
Caseinates are typically dried on a Wide Body dryer with an external fluid bed, fines recycle, nozzles atomisation and provisions for lecithination. Caseinates are very heat stable, excellent fat emulsifiers, and have a high nutritional value. The temperature of the feed to the dryer ranges between 90 – 130 °C, and the inlet temperature of the drying air can be as high as 300 °C for multi-stage drying at an outlet of 90 – 95 °C to obtain a powder moisture content of 4 – 5%.
Application:
- Both sodium and calcium caseinate serve as water binders, emulsifiers, whipping agents, and fillers in food and meat products.
- Protein sources in dry cereals, infant food, dietetic and diabetic products
- Coffee whiteners and toppings
- Meat products to improve texture by binding moisture and fat
For more info about caseinate see Chapter 16.
Milk protein concentrate and isolate (MPC & MPI)
MPC is produced by separating the protein from the other milk components. First the cream is separated from the milk to produce skim milk which then undergoes ultrafiltration to create lactose-reduced skim concentrate. Ultrafiltration separates components based on their molecular size. The UF membrane allows lactose, minerals, and water to pass through while the larger casein and whey protein molecules are retained. This retained liquid is called UF retentate and is further concentrated with membrane filters (ultra/nano) filtration or a falling film evaporator and subsequently dried on a spray dryer to produce MPC powder. Depending on the desired product, different heat treatments can be applied. Since MPC contains heat-sensitive whey protein, which is less heat stable than casein, the heat treatment shall be selected carefully. MPC powders are classified mainly based on the protein content. For instance, MPC70 suggests that the powder contains at least 70% milk protein. MPCs with a protein content of more than 90% are classified as milk protein isolates (MPI).
For more info about filtration see Chapter 7.4.
The maximum total solids of the MPC concentrate depend on the protein level and the temperature at which the concentrate is atomised in the dryer. The higher the protein content in the MPC, the lower the total solids can be due to viscosity limitation. As an indication, depending on the upstream process and required product quality, the concentrate total solids of the different MPCs range from 35 – 40% for MPC40 to 18 – 24% for MPC90 (MPI).
MPC is typically dried multi-stage on a Wide Body spray dryer at an inlet temperature of 200 – 220 °C and an outlet temperature of 75 – 80 °C. Most MPCs are used as ingredients, which does not require the powder to be agglomerated, although this can be an option.
Applications:
- Cheese production: to enhance protein content and improve yield and texture
- Yoghurt and ice cream: to improve texture, mouthfeel, and nutritional profile
- Sports nutrition: used in protein shakes and bars
- Meal replacements: for sustained energy release and muscle maintenance
- Bread and pastries, gluten-free products
- Convenient food: soups, sauces
- Infant formula
Micellar casein concentrate and isolate (MCC & MCI)
In addition to ultrafiltration to produce MPC, the UF retentate undergoes microfiltration (MF) to separate the casein micelles from the whey protein and other milk components. The microfiltration membranes have pores small enough to retain the casein micelles while allowing smaller molecules such as whey proteins, lactose, and minerals, to pass through. Ultrafiltration or nanofiltration is used after microfiltration to further concentrate the casein micelles and remove additional lactose and soluble minerals. Like MPC, MCC is classified by its protein content. MCC with a protein content of more than 90% is classified as micellar casein isolate (MCI). For MCC, similar drying conditions apply. MCC and MCI are typically dried multi-stage on a Wide Body spray dryer.
For more info about filtration see Chapter 7.4.
The permeate from the MF contains high-quality whey proteins that can be further purified and concentrated with ultrafiltration and nanofiltration, and then spray dried to WPI powder, or used in liquid form as ingredient for other products. WPI from milk has significant advantages over WPI from cheese whey. WPI from MF-ed milk contains only native whey protein, is free of fat, has low micro-organism counts, and is free of cheese curd fines and other residues from the cheese process. MCC is characterised by its slow digestion rate, natural micelle structure, high protein content, and richness in essential amino acids.
Application:
- Sport nutrition: slow digestion
- Meal replacements: shakes and bars
- Medical nutrition: steady supply of protein
- Protein supplements: muscle growth
- Functional food
- Cheese production
Whey and whey derivates
Whey ingredients have a relatively short history. Just a generation ago, the whey that was produced during the cheese-making process was considered a waste product. Today, it is regarded as a valuable co-product of cheese making. Whey is refined at the plant into a valuable ingredient with many uses in foods, beverages, and animal feed. Advances in technology and investments in research and development have enabled the whey industry to expand its product line from basic commodities to a variety of higher-valued products, including whey protein concentrates, isolates and fractions. The industry continues to innovate, while at the same time focusing on finding new uses and new markets for these value-added ingredients.
Whey products improve texture, enhance flavour and colour, emulsify, and stabilise, improve flow properties and dispersibility in dry mixes, help extend shelf-life, and exhibit a range of other properties that increase food product quality.
For more info about whey processing see Chapter 18.
Sweet whey powder (SWP)
Sweet whey powder is obtained by drying fresh whey (derived during the manufacture of cheeses such as hard cheese, cheddar, mozzarella, and Swiss), that has been pasteurized and to which nothing has been added as a preservative. Sweet whey powder can be produced either by drying whey concentrate directly from the evaporator or as a pre-crystallised whey concentrate, the latter being the most common process for whey powder production. In both cases, drying is performed single stage.
To produce standard sweet whey powder without pre-crystallisation, the applied conditions are approximately 40 – 45% total solids, a drying air temperature of 160 – 180 °C, and an outlet air temperature of approximately 90 – 95 °C. For pre-crystallised whey powder, the concentrate with ~60% total solids is dried at an inlet air temperature of 180 – 200 °C and an outlet air temperature of 90 – 95 °C.
Pre-crystallised whey concentrate should have an average crystal size of 30 – 50 μ, with a minimum number of crystals larger than 100 μm, and a crystallisation degree of at least 70%, preferably 80%. However, this powder can still be prone to caking and hygroscopic behaviour when exposed to humid air.
To obtain a non-caking and non-hygroscopic product, it is necessary to dry the pre-crystallised product on a dryer concept with a timing belt to allow for sufficient post-crystallisation. A dryer with a crystallisation belt operates at an inlet air temperature of ~160 °C and an outlet air temperature of 55 – 65 °C. The powder leaves the chamber and enters the crystallisation belt with 9 – 12% moisture. A defined residence time of the whey on the belt ensures that most of the remaining amorphous lactose crystallises to non-caking a-lactose-monohydrate. The total solids of the feed from the crystallisation tanks to the dryer ranges between 58 – 62%.
Application:
- Dairy products: serves as an economical source of dairy solids with 8% – 9% salt/ash content.
- Bakery: enhances colour development during high-temperature cooking and baking.
- Snack food: used in chocolate, confectionery, and desserts.
- Ice cream: adds flavour and texture.
- Soups and sauces: used as a thickening agent, providing richness and body.
- Dressings: used for flavour enhancement.
- Pet food: included as a nutritional component.
- Feed products: used in animal feed formulations.
Demineralised whey powder (demin WP)
Demineralised whey powder is produced by removing a significant portion of the minerals from whey, which is a by-product of cheese or casein production. Numbers indicate the percentage of mineral removal, where D30 means 30% demineralisation, D90 means 90% demineralisation, etc. The following methods for removal of the minerals are applied:
- Nanofiltration: This process uses semi-permeable membranes to selectively remove minerals while retaining larger whey proteins and lactose.
- Electrodialysis: An electric field is used to move mineral ions through selective membranes, effectively separating them from the whey.
- Ion exchange: Whey is passed through ion exchange resins that selectively remove cations (e.g. calcium and magnesium) and anions (e.g. chloride and phosphate).
Like sweet whey, demineralised whey is concentrated in an evaporator, pre-crystallised in tanks and spray dried on a Wide Body or Tall Form Bustle dryer to obtain a semi or non-caking product. The drying conditions for demineralised whey are like the conditions for sweet whey. However, pre-crystallisation may require different operating conditions and seeding due to the absence of minerals that act as nuclei for spontaneous crystallisation.
Acid whey powder (AWP)
Acid whey results from the coagulation process used in making acid-coagulated cheeses. It is a co-product obtained during the production of Greek yoghurt, cottage cheese, and cream cheese. When a food-grade acid (such as lactic or citric acid) is added to heated milk, it curdles, separating the milk solids (curds) from the liquid whey. Acid whey has a pH of less than or equal to 5.1 and contains approximately 93% water, 0.8% protein, and 5.1% carbohydrates.
However, drying of acid whey poses several challenges. Acid whey contains higher levels of certain minerals and acids such as calcium, phosphorus and lactic acid compared to sweet whey. This can lead to a more pronounced salty taste, which may not be desirable in all food products. Additionally, high mineral content can affect the powder’s functionality in food formulations. The lower pH of acid whey (around 4.6) compared to sweet whey can impact its stability and taste. The properties of acid whey can complicate the drying process used to produce the powder. Issues such as fouling and clogging in spray dryers can occur more frequently because of high acid content, leading to higher maintenance costs and potential production downtime. To process acid whey, it is recommended to mix the acid whey with sweet whey in a certain ratio to minimise the sticky behaviour of the whey powder during drying.
Like sweet whey, it is possible to dry acid whey without or with pre-crystallisation, although the latter is highly recommendable to obtain a relatively non-caking product. Acid whey can be dried single stage on a Wide Body or Tall Form Bustle dryer at a relatively low inlet air temperature of 160 °C and outlet temperature of 90 – 95 °C. The drying process is very susceptible to changing ambient conditions, especially in areas with high humidity. For this reason, the ambient air is dehumidified to ensure consistent inlet conditions.
Application:
- Feed source for cows
- Co-digestion with manure
- Addition to manure storage structures
Fat-filled whey powder (FFWP)
Fat-filled whey powder is a product derived from whey, which is enriched with vegetable oils or animal fats to increase its fat content. Fat-filled whey powder is often used as an ingredient for stock food and contains 30 – 60% fat. The higher the fat content in the mix, the greater the challenge for the spray dryer, especially regarding powder deposits in the drying chamber, ducting and cyclones. Measures to mitigate the risk of fouling are, amongst others, proper homogenization of the feed, low air velocity profile throughout the dryer, single-stage drying, low inlet air temperature, a static cooling bed, and cooling transport. For fat-filled products above 40%, it is recommended to replace the cyclones with a CIP-able bag filter. For whey with more than 50% fat, it is recommended to apply alternative drying methods. The total solids in the feed increase with increased fat content and can be as high as 60%. Fat-filled whey is typically dried multi-stage on a Wide Body spray dryer at an inlet temperature of ~180 °C and an outlet temperature of 90 – 95 °C.
Application:
- Dairy products: chocolate, ice cream, yoghurt milk drinks, creamer/whitener for coffee and tea
- Bakery and confectionery: biscuits, cream fillings, confectionery
- Food concentrates and dry mixtures: Fat-filled whey powder serves as an excellent fat carrier in various dry food formulations. It contributes to texture, flavour, and stability.
Whey protein concentrate and isolate (WPC, WPI)
Whey protein concentrate (WPC) and isolate (WPI) production involves several stages to extract and purify the protein from whey, a byproduct of cheese manufacturing. As explained previously, WPI can also be extracted from skim milk using ultrafiltration in combination with microfiltration, which provides a higher-grade whey protein powder.
After clarification, separation, and pasteurization (75 °C for 15 sec), the cheese whey undergoes ultrafiltration to separate the whey protein from water, lactose, and minerals. The concentrated whey proteins (WPC) at this stage still contain residual fat from cheese production. Diafiltration can be used to further purify the protein concentrate by washing out additional lactose and minerals. In this case, water is added to the retentate and the mixture is passed through the UF membranes again. The concentrated protein (UF retentate) is further concentrated through nanofiltration or evaporation to reduce water content.
To produce WPI, the UF retentate shall undergo microfiltration to separate the residual fat from the protein. Subsequently, the proteins (MF permeate) are further purified and concentrated with ultrafiltration and nanofiltration to the maximum achievable total solids. The byproduct from microfiltration (MF retentate) is a WPC with high fat content (15 – 20%), also known as whey protein phospholipid concentrate (WPPC), which can also be spray dried.
WPC powders are classified mainly based on the protein content. For instance, WPC85 suggest that the powder contains at least 85% whey protein. WPC with a protein content of more than 90% is classified as whey protein isolate (WPI) with a lactose content of less than 1% and less than 1.5% fat.
The maximum total solids achievable for WPC and WPI is subject to the protein content. The higher the protein content in the WPC, the lower the total solids due to viscosity limitation. As an indication, depending on the upstream process and required product quality, the concentrate total solids of the different WPCs range from ~45% for WPC35 and ~36% for WPC80.
WPC and WPI can be dried single or multi-stage on a Wide Body or Tall Form Bustle spray dryer at an inlet temperature of 200 – 240 °C and an outlet temperature of 75 – 85 °C. The selected type of dryer is sometimes dictated by the customer. Some producers claim that the Tall Form Bustle dryer provides less thermal load on the product. Due to their heat sensitivity, WPC and WPI concentrates are usually spray dried cold at ~10 °C. Pasteurization of WPC and WPI can be performed but preferably at relatively low total solids to prevent denaturation of the heat-sensitive whey protein. Depending on the application, the WPC powder can be instantised (agglomerated + lecithinated) or regular, non-agglomerated.
In the case of WPI production, there is an option to also dry the WPPC (byproduct from MF) on the same dryer. WPI and WPPC are dried alternately which requires the capacity and size of the spray drier to be designed accordingly to cater for enough time for CIP and change-over. One of the challenges in such a case is to prevent contamination between products, especially WPI contamination.
Application:
- WPC is versatile and commonly used in various food products, sports nutrition, and nutraceuticals. It is a cost-effective source of protein and is used to enhance the nutritional profile and texture of foods.
- WPI is preferred for applications requiring higher protein purity and minimal lactose, such as products for lactose-intolerant consumers, high-performance sports nutrition, and specialised medical nutrition. It is also used in clear beverages and hypoallergenic infant formulas due to its low allergen potential.
Whey protein fractions (α-lac, β-lac, lactoferrin, etc.)
Whey protein contains several key protein fractions, each with unique properties and benefits. The production of these whey protein fractions involves several steps to isolate and concentrate the different protein components from whey and to ensure purity and functionality. This not only enhances the nutritional and functional properties of whey proteins but also allows for their application in a wide range of nutritional and therapeutic products. Table 19.8 lists the primary protein fractions in whey.
Overview of key processes for whey protein fractions:
- Clarification and separation for removal of cheese fines and whey cream
- Ultrafiltration and microfiltration for concentration of whey protein by removal of lactose and minerals (UF) and separation of fat (MF)
- Ion exchange chromatography for the separation of proteins based on their charge. The whey proteins are passed through a column containing charged resin beads, which bind specific proteins. By changing the pH or ionic strength of the solution, different proteins can be selectively eluted (released) from the column.
- Enzymatic hydrolysis for breaking down proteins into peptides using specific enzymes. This process improves the digestibility and bioavailability of proteins. It is also used to produce protein hydrolysates with specific functional properties.
For more info about ion exchange see Chapter 18.
Applications:
- Sports nutrition: high in BCAAs and other essential amino acids, promoting muscle repair and growth
- Medical nutrition: used in formulations for individuals with specific dietary needs, such as those with PKU (GMP-rich whey) or compromised immune systems (IgGs and LF-rich whey)
- Functional foods: added to foods to enhance nutritional value, improve texture, and provide health benefits
- Infant formula: mimics the protein composition of human milk, particularly a-lactalbumin, to support infant growth and development
Whey permeate powder (WPP)
A by-product in the manufacture of whey protein concentrates and isolates is whey permeate. Whey permeate consists mainly of lactose and salts but also may include relatively small quantities of monosaccharides like glucose and galactose, and acids like lactic and citric acid. These constituents are known to have an adverse effect on the crystallisation and dry ability of the concentrate due to their low glass transition temperature. This could result in a whey permeate powder which is more caking and/or hygroscopic if the process is not selected carefully. The composition of whey permeate depends largely on the type of cheese. Whey permeate from semi-hard cheese contains less sugars and acids compared to, for instance, mozzarella cheese which usually contains higher quantities of acids and mono sugars. Pre-treatment of the whey permeate with nanofiltration (NF) could reduce the level of sugars and acids which will be beneficial for dryability. Nanofiltration, however, will create an effluent stream (NF permeate) that must be managed.
Whey permeate can be dried with similar technology as non-caking whey powder. To achieve a non-caking and non-hygroscopic product, it is necessary to pre-crystallise the product in crystallisation tanks prior to drying. The pre-crystallised product can be dried both single and multi-stage on a Wide Body or Tall Form spray dryer. To obtain a non-caking and non-hygroscopic product, it is necessary to dry the pre-crystallised product on a dryer concept with a timing belt to allow for sufficient post-crystallisation.
If a semi-caking product is acceptable, single-stage drying will suffice to keep the capital cost relatively low.
A single-stage dryer typically operates at an inlet temperature of 170 – 180 °C and an outlet air temperature of 90 – 95 °C. The total solids of the pre-crystallised permeate shall not be higher than 58 – 60% to ensure low viscosity hence efficient drying. The whey permeate will be partially caking but if the powder is packed and sealed properly, it should remain non-caking for an extended period. Quick handling of the powder after opening of the bag however is recommendable to minimise the risk of lump formation.
A dryer with a crystallisation belt operates at an inlet air temperature of ~160 °C and an outlet air temperature of 55 – 65 °C. The powder leaves the chamber and enters the crystallisation belt with 9 – 12% moisture. A defined residence time of the whey on the belt ensures that most of the remaining amorphous lactose crystallises to non-caking a-lactose-monohydrate. The total solids of the feed from the crystallisation tanks to the dryer ranges between 62 – 66%.
Application:
- Bakery products and pizza crust dough: Whey permeate can be used as a direct replacement for other dairy solids in bakery goods, contributing to browning crusts and enhancing flavour.
- Confectionery products: It provides mild sweetness and a pleasant milky flavour in confectionery items.
- Bio fermentation: It is utilised in fermentation processes to produce energy.
- Animal feed: High-purity lactose is not always required, making whey permeate suitable for animal feed applications.
- Food additive: Whey permeate acts as a free-flowing bulking agent that dissolves quickly.
- Other applications: Whey permeate is a cost-competitive source of dairy solids, making it versatile across various industries. Additionally, it’s used in chocolate, sauces, and seasoning due to its sweet dairy flavour.
Lactose powder (lactose)
Lactose powder is a refined form of lactose, a disaccharide sugar derived from milk. Depending on the milk source, lactose makes up about 40 – 55% of the total solids. Lactose in solution is available as a-lactose and β-lactose in a fixed ratio. Lactose solubility is relatively low but below 95 ºC a-lactose is less soluble than β-lactose. Lactose crystallises at ambient temperatures as a-lactose monohydrate. On dry solids, the amount of crystal water is 5%. Lactose is an important ingredient in many nutritional formulations and is a popular filling agent in (pharma) tablets.
The raw materials for lactose manufacturing are cheese and casein whey, and whey permeates. To produce lactose, whey permeate is desalted and decalcified with nanofiltration and a decalcifying process. The desalted permeate is then concentrated to 65% in an evaporator after which it is crystallised to form large crystals. The lactose slurry is subsequently concentrated in a decanter and sieve centrifuged, after which the purified crystallised lactose is dried in a fluidising bed. The purity of the lactose from this process is typically ~99.7%. For higher purity additional process steps are required to remove remaining minerals and organic acids. Active carbon can be used to remove riboflavin responsible for the yellow colour of the lactose.
The fluid bed for drying and cooling of the lactose is divided into multiple sections. The lactose slurry that is pumped to the inlet of the fluid bed dryer contains ~90% total solids. The drying sections operate at an inlet temperature of 150 °C. During drying, the temperature of the lactose powder increases to approximately 110 °C which ensures that all the free water is removed. In the cooling section, the powder is cooled down to ~25 °C. The crystallised lactose powder contains about 5% bound water.
Application:
- Food industry: bakery, confections, snacks, frozen desserts, sweeteners, meat products, soups and sauces, beer production, nutraceuticals
- Pharmaceuticals and nutritional supplements: tablets and capsules, dry powder inhalers
- Infant nutrition: Lactose is a staple in baby formulas, from infant to toddler stages.
- Lactose derivatives: Lactose serves as a substrate for producing compounds like GOS (galactooligosaccharides), lactulose, and HMO (human milk oligosaccharides).
- Chocolate industry: Lactose contributes to a soft taste and cohesive texture in chocolate, improving crystallisation.
Baby food
The main constituents in baby food and other nutritional formulas are proteins, carbohydrates, and fats. Essential minerals, trace elements and vitamins are added to complete the nutritional requirements. The technology for preparing specific formula recipes remains proprietary to the manufacturer.
Suppliers of baby food powder aim to mimic the composition of human milk to ensure that infants receive the necessary nutrients for healthy growth and development. Typical ingredients in baby food powders are:
Macro ingredients:
- Proteins: Human milk contains whey and casein proteins. Baby food powders aim to balance these proteins, typically with a higher proportion of whey to casein to match early human milk.
- Fats: Human milk contains essential fatty acids, including DHA and ARA, which are crucial for brain and eye development. Baby formulas often add these fats to closely resemble human milk.
- Carbohydrates: Lactose is the primary carbohydrate in human milk, providing energy and aiding in calcium absorption. Most baby formulas use lactose as the main carbohydrate source.
Micro ingredients:
- Vitamins: Formulas are fortified with vitamins A, D, E, K, C, and B-complex to ensure infants get the necessary nutrients for immune function, bone health, and overall growth.
- Minerals: Essential minerals like calcium, phosphorus, magnesium, iron, zinc, and iodine are added to support bone development, oxygen transport, and metabolic processes.
Bioactive ingredients:
- Nucleotides: These are added to support immune function and intestinal development.
- Probiotics and prebiotics: To promote a healthy gut microbiome, formulas may include beneficial bacteria (probiotics) and food for these bacteria (prebiotics).
- Human milk oligosaccharides (HMOs): These complex carbohydrates found in human milk support gut health and immune function. Some advanced formulas now include synthetic HMOs.
All nutritional formulations are characterised by high carbohydrate levels, typically ranging from 55 to 70%. Carbohydrates like lactose, sucrose and glucose syrups are the constituents responsible for stickiness in spray drying. Considering the sticky nature of almost all nutritional formulations, single-stage spray drying is applied. This means drying at a relatively "low" product moisture in the spray dryer which is typically between 2.5 and 3.5%.
Due to the wide variety of baby powder compositions, the concept and operating conditions of the spray dryer must be selected carefully. Certain infant formulations contain constituents like GOS or FOS or are hydrolysed, contributing to the sticky behaviour of the product during drying. Therefore, dryer concepts are tailored to suit the requirements.
Baby food powders are categorised into different stages based on age, with each stage having its own unique composition. The composition of baby food powder for infants in stage 1 (0 – 6 months) is carefully formulated to meet the nutritional needs of very young infants who are just starting solid foods. These products are designed to be easily digestible and to provide essential nutrients required for healthy growth and development. In general, the powder intended for younger ages tends to have higher sugar content and lower protein levels. This can pose challenges during the drying process. For each composition, a sticky/drying curve is prepared to determine the dryer operating conditions.
In the spray drying process for baby food, climatic conditions play a crucial role. To mitigate their impact, manufacturers commonly dehumidify the ambient air. This practice helps to ensure efficient drying, maintain consistent product quality, and elongate dryer runtime.
To meet these criteria the Wide Body spray dryer with integrated static, or external well-mixed bed, fluid bed for cooling, cyclones, and CIP-able bag filter is the most suitable concept. Fines from the cyclones collected are conveyed by means of a pneumatic transport system to the atomisation zone for agglomeration. In the case of extremely thermoplastic product, the fines can be collected, conveyed and cooled in a so-called open transport cooling system to mitigate the risk of blockages.
The drying air inlet temperature for baby food powder production is in the range of 160 – 190 °C and an outlet air temperature of 85 – 95 °C. The concentrate feed temperature is 75 – 80 °C. The concentrate feed to the dryer is typically two-stage homogenized at 100 – 120 and 20 – 40 bar.
Milk powder packing
Milk powder packing involves several critical steps to ensure the product's quality, safety, and shelf life. The process is designed to protect the powder from moisture, oxygen, light, and contamination. Commonly used packing types for milk powders are 25 kg bags or 1,000 kg big bags.
Key considerations:
- Moisture barrier: prevents moisture ingress, which can lead to caking and spoilage
- Oxygen barrier: reduces oxidation and rancidity, preserving the flavour and nutritional quality
- Light protection: prevents degradation of sensitive vitamins and proteins
Packaging types
Bags and pouches
- Multi-layer bags: typically made of kraft paper with inner layers of polyethylene or aluminium foil for moisture and oxygen barrier properties
- Plastic bags: high-density polyethene (HDPE) or low-density polyethene (LDPE) bags for smaller quantities
- Resealable pouches: flexible plastic or laminated pouches with resealable zippers, often used for consumer-friendly packaging
Cans and tins
- Metal cans: often lined with a protective coating to prevent interaction between the powder and the metal. They provide excellent protection from light, moisture, and oxygen.
- Composite cans: made from layers of paper, plastic, and foil, offering similar protection with less weight
Boxes and cartons
- Fibreboard boxes: used for bulk packaging with an inner plastic lining for moisture protection
- Cartons: multi-layer cartons with aluminium and plastic lining, commonly used for consumer-sized packages
Bulk containers
- Big bags (FIBC – flexible intermediate bulk containers): large, woven polypropylene bags used for industrial-scale quantities
- Drums: metal or plastic drums for large quantities, often used in industrial applications
Powder characteristics
Achieving the desired properties in milk powder involves several key steps and considerations in the manufacturing process. The quality and composition of the milk used (e.g. cow's milk, goat's milk, sheep's milk, etc.) is one element that influences the final properties of the milk powder. Factors such as fat content, protein content, and lactose levels are important considerations. Since certain factors are susceptible to seasonal and daily fluctuations, it is essential to monitor these properties. This ensures that any variations caused by these fluctuations are identified and that the operational parameters are adjusted accordingly. Effectively, powder characteristics can be categorised into 3 groups: chemical, physical, and functional. In Table 19.10, a selection of typical powder characteristics are listed. Depending on the type of powder, moisture content, scorched particles, bulk density and flowability probably receive the most attention during milk powder production.
Moisture content
The moisture content in milk powders is an important parameter as it affects the powder's shelf life, quality, and reconstitution properties. The moisture content is the amount of water contained in the powder expressed in percentage weight. Maintaining low moisture content is crucial for preventing microbial growth and ensuring a long shelf life for milk powders.
The global standards for moisture content in milk powder are typically set by various food safety and quality organisations. Two prominent standards are from the Codex Alimentarius and the European Union. The final moisture content is important from the point of view of final powder quality and achieving a standard product. From an economical point of view, it is important to operate as close as possible to the limit. In large spray drying installations, each 0.1% of moisture can represent a great sum of money on a yearly basis. However, no less important is the intermediate moisture content of a product leaving the individual processing steps during two-stage or three-stage drying. The intermediate moisture content has a great influence on such properties as solubility index, bulk density, particle density, agglomeration (i.e. particle size distribution) and overall drying economy.
Scorched particles
Scorched particles in milk powder refer to darkened or burnt fragments that result from excessive heat during the processing or drying stages of milk powder production. These scorched particles can negatively impact the quality and appearance of the milk powder. Scorched particles in milk powder can also have safety implications primarily related to potential chemical and microbiological changes (Maillard reaction) that may occur due to the overheating or burning of milk solids.
Scorched particles are measured by filtering a solution of 250 ml water with 25 g skim milk powder, or 32.5 g whole milk powder through a filter pad. The result is assessed using the standard scale developed by the ADPI (American Dairy Product Institute).
Bulk density
Bulk density is a critical parameter for milk powders because it influences various aspects of their handling, processing, packaging, and application. The bulk density of milk powder can vary depending on its type, production method, and particle size. Bulk density is typically expressed in units of mass per unit volume, g/cm3, kg/m3 or in g/100 ml. Generally, the bulk density of milk powder ranges between 0.30 to 0.80 g/cm³.
Bulk density is measured by loosely filling a glass cylinder with a volume of 250 ml with 100 g of powder. By automatically tapping the filled glass with an apparatus the bulk density can be determined at 100, 200, 600 and 1,250 taps.
Bulk density is a very complex property and depends on many other properties, such as but not limited to particle density, occluded air, morphology, etc., which in turn depend on the dryer concept and drying process conditions.
Flowability
Flowability refers to the ability of milk powder to flow freely and smoothly under various conditions, such as during handling, processing, and packaging. It is an important property that impacts the practical aspects of using milk powder in industrial processes and consumer applications. Key aspects related to the flowability of milk powder are particle size distribution, particle shape, moisture content, bulk density, occluded air, etc.
The flowability of milk powder, like other powdered materials, can be measured and tested using several methods that assess different aspects of its behaviour under various conditions. Some common techniques used to measure and test the flowability of milk powder are angle of repose, flowability index, shear cell testing and powder rheometry.
Energy economy of spray drying
For the spray drying process, thermal energy is indispensable to obtain a product that is commercially dry but also meets functional requirements. Spray drying has proven to be the most suitable process but is also acknowledged as an energy-intensive process where a considerable amount of heat is disposed into the atmosphere in the form of warm and moist exhaust air. There are, however, techniques that can be applied to minimise the energy cost.
These include:
- Minimising the water content of the feedstock prior to spray drying
- Maximising the temperature drop of the drying air, i.e. between the maximum inlet and minimum outlet temperature (Tin-Tout)
- Multi-stage drying
- Utilising the heat in the exhaust air to pre-heat the incoming drying air
- Utilising surplus of heat from other unit operations
- Reducing radiation and convection heat loss by means of thermal insulation
- Efficient atomisation and dispersion of product in the drying air
Water content
The most efficient way to save energy is to minimise the water content of the concentrate feed prior to spray drying by pre-treatment with other techniques like mechanical separation and/or evaporation. Mechanical separation processes like filtration are far more energy efficient than thermal processes. When mechanical separation is not possible, evaporation should be considered as it provides a far more efficient process than spray drying. Falling film evaporators are known to be over ten to thirty times more efficient than spray dryers. Often the limiting factor for the removal of water in a falling film evaporator is the viscosity of the concentrate.
Drying air temperature profile
In falling film evaporators, the latent heat of the evaporated water is reused in subsequent stages downstream, which operate at a lower pressure and temperature. The vapour contained in the air from a spray dryer is not easily recovered apart from some preheating applications that reduce the thermal potential. Therefore, it is important to minimise the volume of drying air used to transport the heat and carry over the vapour that is generated. If large quantities of air exit the dryer, an equally large amount of heat is lost. The higher the inlet air temperature, the lower the amount of air required, and the higher the efficiency of the dryer. There are, however, temperature limitations associated with the product which limit both the inlet and outlet air temperature. Often the inlet and outlet temperatures are dictated by the composition of the product and the thermoplastic behaviour thereof. Table 19.11 shows the energy efficiency of two products dried under different conditions.
Multi-stage drying
Two- or three-stage dryers allow the powder to leave the drying chamber at higher residual moisture content, thus at a lower outlet air temperature. This, in turn, improves the energy efficiency, as the difference between the inlet and outlet temperature is higher, resulting in a higher efficiency. In addition, multi-stage drying allows for higher inlet air temperature, making it even more efficient. Table 19.11 shows an example of the dryer's thermal efficiency for single- and multi-stage dried products.
Heat recuperation
Heat recuperation in a spray dryer refers to the process of recovering heat from the hot gases exiting the dryer. This is typically done to improve energy efficiency and reduce operational costs. Heat recovery generally works as follows:
- Hot gas recovery: As the hot exhaust gases leave the spray dryer, they still contain a significant amount of thermal energy. Instead of releasing this heat into the atmosphere, it can be captured and reused.
- Heat exchanger: A heat exchanger is usually employed to transfer the heat from the exhaust gases to another medium, such as incoming drying air or water. The recovered heat raises the temperature of the incoming medium, reducing the overall energy input needed to achieve the desired drying conditions.
- Energy efficiency: By recuperating heat, the overall energy efficiency of the spray drying process improves. This can lead to lower energy consumption and operational costs.
- Design considerations: Engineers must consider the design of the heat exchanger and the integration with the existing spray drying system. Factors such as type of heat exchanger (e.g. plate heat exchanger, tubular heat exchanger), hygiene, and operating conditions are crucial to maximise heat recovery efficiency while ensuring food safety. One of the risks associated with exhaust heat recovery is the fouling of the heat exchanger due to humid conditions that may result in potential bacteriological issues.
- Environmental impact: Improved energy efficiency also translates to reduced greenhouse gas emissions and environmental impact associated with energy consumption.
Overall, heat recuperation in a spray dryer is a significant aspect of optimising industrial drying processes, ensuring they are both economically viable and environmentally sustainable.
Energy can also be saved at various points throughout the production process. For example, hot condensate from the evaporator can be cooled in the pre-heater of the spray dryer. Heat exchangers can also take advantage of high-temperature combustion gas from a gas or oil heater to pre-heat the air intake to the spray dryer.
A heat recuperation system is typically installed after the bag filter to minimise the formation of deposits on the surface of the heat exchanger. However, some particles will always be contained in the exhaust air after a bag filter which may adhere to the surface of heat exchangers. For this reason, the recuperator is usually CIP-compatible.
In an air-to-liquid recuperator, a liquid is heated in a heat exchanger installed in the exhaust air duct. The liquid is pumped and circulated through a pre-heater placed in the inlet air duct where the energy is utilised for pre-heating the dryer inlet air.
Whether heat recuperation is economically attractive depends on the amount of residual energy in the exhaust air. This is often dictated by the type of product that is dried. Products with a high thermo-plasticity need to be dried at a relatively high outlet air temperature. Baby food products for instance are dried at an outlet air temperature of 90 – 95 °C, making the investment in a heat recuperator economically feasible. Recuperation can save up to 20% of the dryer's energy consumption.
Sustainability
Sustainability in spray drying involves implementing practices and technologies that minimise environmental impact, conserve resources, and optimise energy efficiency throughout the drying process. Key aspects of sustainability in spray drying are:
- Energy efficiency: Efficient use of energy is crucial in spray drying. This includes technologies like heat recuperation systems, which recover heat from exhaust gases to preheat incoming air or water. Optimising insulation and airflow management also contribute to energy efficiency.
- Resource conservation: Minimising water usage and waste generation is essential. Techniques such as closed-loop systems for water recycling and waste minimisation strategies help reduce environmental footprint.
- Raw material utilisation: Efficient use of raw materials, such as optimising powder recovery and minimising product losses, contributes to sustainability. This reduces material waste and improves overall process efficiency.
- Emissions reduction: Controlling emissions and pollutants from the drying process is critical. This involves using efficient air filtration systems and adopting technologies that reduce volatile organic compounds (VOCs) and other harmful emissions.
- Renewable energy: Incorporating renewable energy sources such as electricity from solar and wind power, into spray drying operations further enhances sustainability by reducing reliance on fossil fuels and lowering greenhouse gas emissions.
- Innovation and research: Continuous research and development in spray drying technologies aim to improve efficiency, reduce energy consumption, and explore alternative materials and processes that are more environmentally friendly. As such, electrical air heating systems and heat pump technology are increasingly used
By integrating these sustainability principles into spray drying processes, industries can achieve not only environmental benefits but also economic advantages through reduced operational costs and enhanced market competitiveness in a world increasingly focused on sustainable practices.