CASEIN

Casein is the major protein in cows’ milk, and comprises about 80 % of the total protein content of which the rest,
some 20 %, are the whey or serum proteins.
Casein is the basic component of ordinary cheese. In the cheese-making process, casein is precipitated by the action of rennet enzymes, and a coagulum is formed consisting of casein, whey proteins, fat, lactose and the minerals of the milk.
Commercial casein is made from skim milk by one of two general methods – precipitation by acid or coagulation by rennet. As much of the fat, whey proteins, lactose and minerals as possible must be removed by multistage washing in water, as they reduce the quality of the casein as well as its keeping quality. Dried, properly produced casein has a relatively good keeping quality and is used mainly in the food and chemical industries.
Types of casein
Casein is usually divided into the following types:
- Rennet casein, obtained by enzymatic precipitation
- Acid casein, obtained by acidifying skim milk to the isoelectric point (pH 4.6 – 4.7)
In addition to these two main types, there are other commercially available casein products of importance, such as:
- Co-precipitate, made by heating skim milk to a high temperature and then precipitating the casein/whey protein complex, usually with calcium chloride.
The co-precipitate also contains whey proteins and calcium.
- Caseinates, commonly sodium caseinate, obtained from acid casein dissolved in sodium hydroxide
Influence of raw material
In order to produce high-quality casein, the raw material, skim milk, must be of good quality. If bacteria have had time to act on the protein in the milk as a result of a change in acidity, this will affect the colour and consistency of the casein, which will acquire a greyish colour and a smoother consistency. Excessive heating of the milk before precipitation will not only cause assorted interactions among the lactose, casein and whey protein constituents, but it will also give the casein a yellow or at worst a brownish colour.
In order to produce casein of good bacteriological quality, without high heat treatment of the skim milk, the pasteurization plant may also contain a microfiltration (MF) plant. To satisfy the high demands on the quality of casein intended for use in the food industry, not only must the production line be carefully planned right from the reception of the milk, but the treatment and handling of the raw material prior to this stage must also be carefully controlled.
Rennet casein
Skim milk, normally pasteurized at 72 °C for 15 – 20 seconds, is used for the production of rennet casein, as well as other types of casein. Small amounts of fat are detrimental to the quality. It is therefore important that the milk has been separated efficiently.
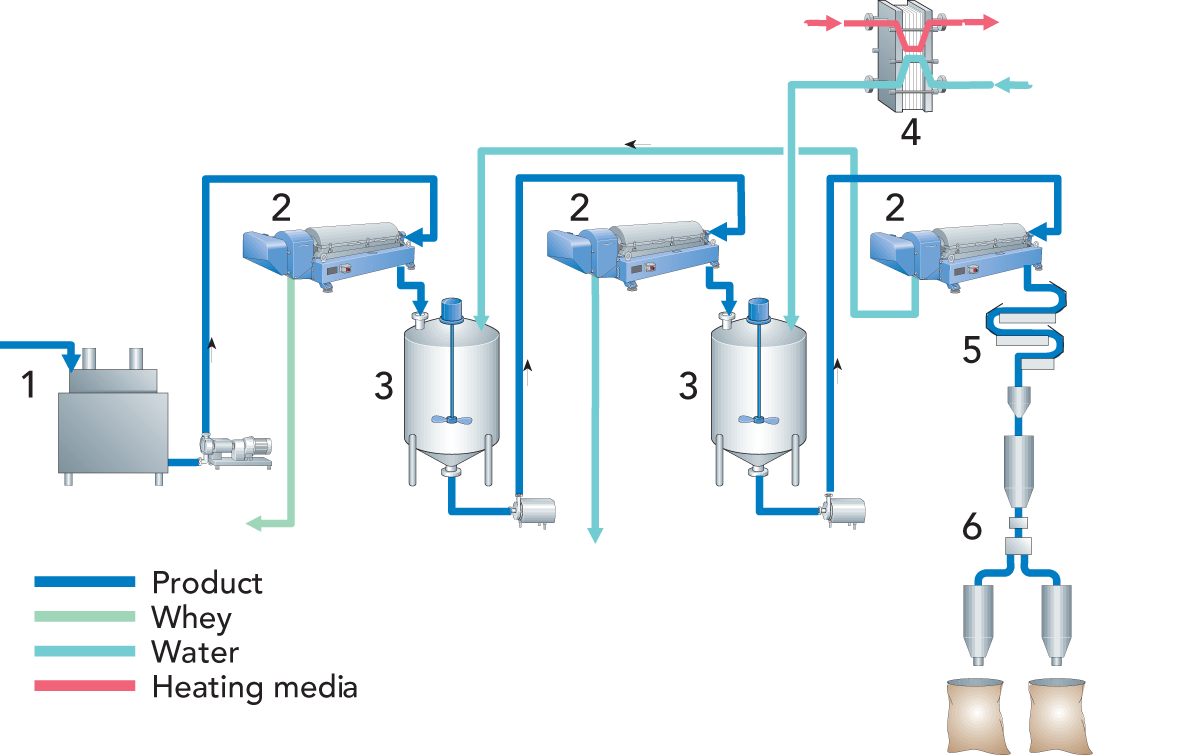
Figure 20.1 shows the various stages of rennet casein production. Renneting takes place with the help of the enzyme chymosin in the rennet. The milk is heated for a short period of time and then cooled to about 30 °C. Then the rennet is added. A gel forms after 15 – 20 minutes. It is cut and the coagulum is stirred while being heated to about 60 °C. The high temperature is needed to deactivate the enzyme. Cooking time is around 30 minutes.
Batch washing
The whey is drained off when the final temperature has been reached, and the remaining casein, while in the vat, is washed with water to remove whey proteins, lactose and salt. Washing takes place in two or three stages at a temperature between 45 and 60 °C.
After the water has been drained off, the casein is further dewatered in sieves or separators. It is then dried with hot air until the water content is
12 %, and finally ground to a powder. The drying temperature depends on the method used. In a two-stage drying process, the temperature is 50 – 55 °C in the first stage and about 65 °C in the second.
Rennet casein should be white or slightly yellow. A darker colour is a sign of inferior quality and may be caused by too high a lactose content.
Continuous washing
Rennet casein was originally produced in batches in special casein tanks, but nowadays continuous processes are also used. In a continuous plant, drainage of whey takes place before the casein passes through two or three washing tanks with agitators. Dewheying is normally done in a decanter centrifuge to reduce consumption of wash water. The casein is dewatered between washing stages, either on inclined static strainers or in decanters. After leaving the washing stages, the water/casein mixture goes through another decanter to discharge as much water as possible before final drying.
In large scale production, coagulation of the casein is still done batchwise with a calculated number of casein vats emptied in sequence to feed the continuous de-wheying and washing plant.
Washing takes place in countercurrent, which uses water more economically than concurrent washing. The latter system uses one litre of water per litre of skim milk, whereas only about 0.3 – 0.4 litre of water per litre of skim milk is needed in countercurrent washing. The number of washing stages is dependent on the requirements on the product. Two stages is the minimum. Fresh water is supplied in the last stage only. After washing, the casein is dewatered in a decanter to a DM content of 45 – 40 %. After drying, for example in a vibration dryer, the casein is ground to a particle size corresponding to 40, 60, or 80 mesh and packed in sacks. (Mesh = number of screen lines per inch; 40 mesh thus corresponds to 0.64 mm.)
Acid casein
The milk is acidified to the isoelectric point of casein, which is thought to be pH 4.6, but it is shifted by the presence of neutral salts in solution and may be anywhere within a range extending from pH 4.0 to pH 4.8. The isoelectric point is the stage where the hydronium ion concentration neutralizes the negatively charged casein micelles, resulting in precipitation (coagulation) of the casein complex. Such acidification can be carried out biologically or by the addition of a mineral acid, e.g. hydrochloric acid (HCl) or sulphuric acid (H2SO4).
Biological acidification – lactic acid casein
Lactic acid casein is produced by microbiological acidulation. The milk is pasteurized and cooled to 27 – 23 °C. A mesophilic, non-gas-producing starter is then added. Acidulation to the required pH takes about 15 hours. If the acidulation process is too rapid, it can result in problems such as uneven quality and reduced casein yield. Large tanks are usually used, because it can take such a long time to empty the tank, that the degree of acidity may vary.
When the required acidity has been reached, the milk is stirred and heated to 50 – 55 °C in a plate heat exchanger. After a short hold, the continued treatment – washing and drying – is practically the same as for rennet casein.
Mineral acidification – acid casein
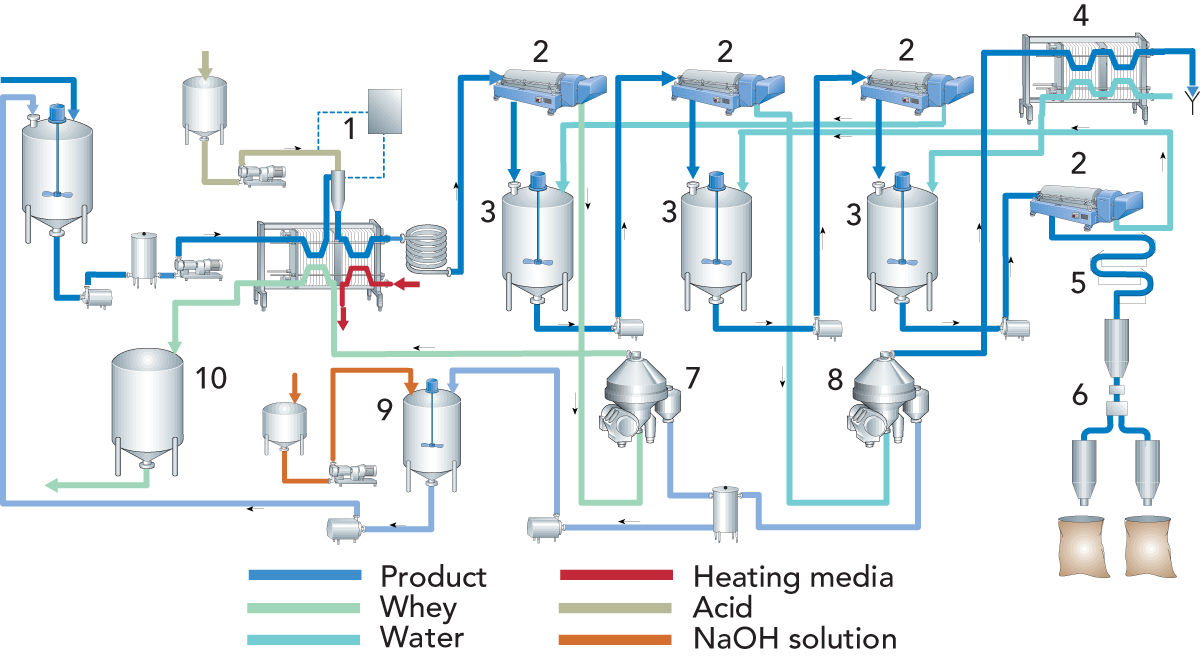
The milk is heated to the required temperature, approx. 32 °C. Mineral acid is then added to bring the pH of the milk to 4.3 – 4.6. Following the pH check, the milk is heated to 40 – 45 °C in a plate heat exchanger and held for about two minutes, when smooth aggregates of casein are formed. To remove as much of the whey as possible before washing starts, the whey/casein mixture is passed through a decanter. This way, less water is needed for washing.
Figure 20.2 shows a flow chart for a process line for the manufacture of acid casein. As can be seen, the plant downstream of acidification is almost identical to the one used for production of rennet casein.
Before leaving the plant, the whey and wash water can be separated and the casein sludge is collected in a tank. When mixed with a lye solution, the casein dissolves and is then remixed with the skim milk intended for casein production.
After dewatering, the acid casein is ground and packed in sacks.
The technique for production of acid casein developed by Pillet, France, should also be mentioned.
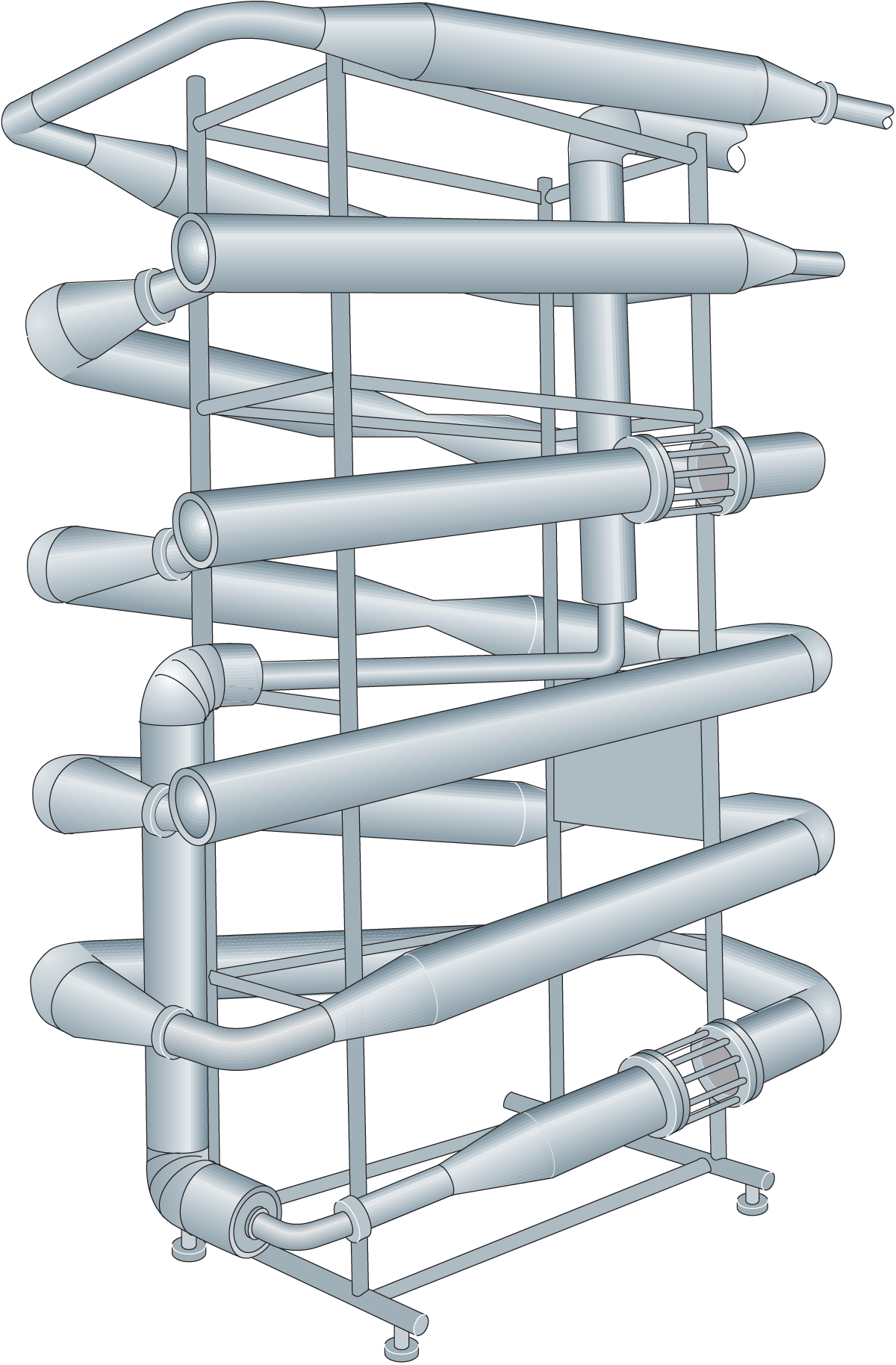
After pre-heating to 32 °C, the skim milk is acidified and introduced into a coagulation unit (Figure 20.3). Coagulation is completed after heating to about 45 °C by direct steam injection. Dewheying in a decanter is followed by countercurrent washing in one or two specially designed washing towers (Figure 20.4).
Before being dried in a vibro-fluidized unit, the casein is dewatered in a decanter.
Co-precipitate
Co-precipitate contains practically all the protein fractions of milk.
Following the addition of small quantities of calcium chloride or acid to the skim milk, the mixture is heated to 85 – 95 °C and held at that temperature for a period of 1 – 20 minutes to allow interaction between the caseins and the whey proteins. Precipitation of the proteins from the heated milk is then effected by controlled addition of either calcium chloride solution (to produce high-calcium co-precipitate) or diluted acid (to produce medium-calcium or low-calcium co-precipitate, depending upon the amount of acid added and the pH of the resulting whey). The curd is subsequently washed and either dried to produce granular, insoluble co-precipitates or dissolved in alkali as described for the methods for the manufacture of caseinates to produce soluble or “dispersible” co-precipitates.
Caseinate
Caseinate may be defined as a chemical compound of casein and light metals, e.g. monovalent sodium (Na+) or divalent calcium (Ca++).
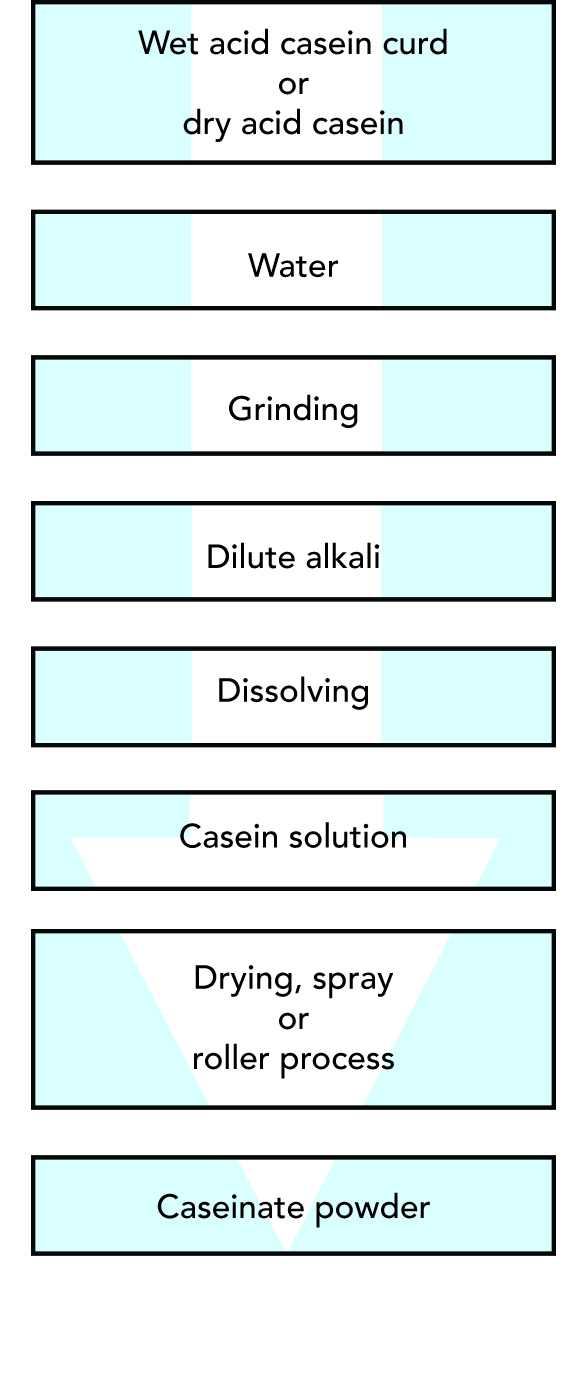
Caseinates can be produced from freshly precipitated ("wet") acid casein curd or from dry acid casein by reaction with any of several diluted solutions of alkali as outlined in Figure 20.5.
Sodium caseinate
The most commonly used alkali in the production of sodium caseinate is sodium hydroxide (NaOH) solution, with a strength of 2.5 M or 10 %. The quantity of NaOH required is generally 1.7 – 2.2 % by weight of the casein solids in order to reach a final pH, generally about 6.7.
Other alkalis, such as sodium bicarbonate or sodium phosphates, may be used, but the amounts required and their cost are both greater than those of NaOH. They are therefore generally used only for specific purposes, such as in the manufacture of citrated caseinates.
The very high viscosity of sodium caseinate solutions of moderate concentration limits their solids content for spray drying to about 20 %.
Regarding the processing procedures, it should be mentioned that the dissolving time is directly related to the particle size and that particle size reduction prior to addition of sodium hydroxide rather than afterwards produces a more rapid reaction. Consequently, the curd is passed through a colloid mill prior to addition of alkali.
After the final casein wash, the curd may be dewatered to about 45% solids and then remixed with water (to 25 – 30% solids) before entering the colloid mill. The temperature of the emerging slurry should be below 45 °C since it has been observed that milled curd can re-agglomerate at higher temperatures. Generally the slurry is collected in a jacketed tank provided with an effective agitator and also integrated in a circulation system with a high capacity pump.
The addition of diluted alkali must be carefully controlled with the aim of reaching a final pH of about 6.7. Preferably, the alkali is dosed into the recirculation line just upstream of the pump.
Once the alkali has been added to the slurry, it is important to raise the temperature as quickly as possible to 60 – 75 °C, to reduce the viscosity.
The dissolving time for sodium caseinate prepared in batches is usually 30 – 60 min.
For efficient atomization, the sodium caseinate solution must have a constant viscosity when it is fed to the spray drier. It is common practice to minimize the viscosity by pre-heating the solution to 90 – 95 °C just prior to spray drying.
Calcium caseinate
The preparation of calcium caseinate follows the same general lines as for sodium caseinate, with a couple of important exceptions. Calcium caseinate solutions are liable to be destabilized by heating, especially at pH values below 6.
It has been found that during the dissolving process, the reaction between acid casein curd and calcium hydroxide proceeds at a much slower rate than between curd and sodium hydroxide. To increase the rate of reaction between casein and calcium hydroxide, the casein may first be dissolved completely in ammonia. Calcium hydroxide in sucrose solution is then added, and the calcium caseinate solution is dried on rollers. Most of the ammonia evaporates during this process.
Other caseinates
Magnesium caseinate has been briefly mentioned in the literature.
Compounds of casein with aluminium have been prepared for medical use or for use as an emulsifier in meat products.
Heavy metal derivatives of casein which have been used principally for therapeutic purposes include those containing silver, mercury, iron and bismuth. Iron and copper caseinates have also been prepared by ion exchange for use in infant and dietic products.
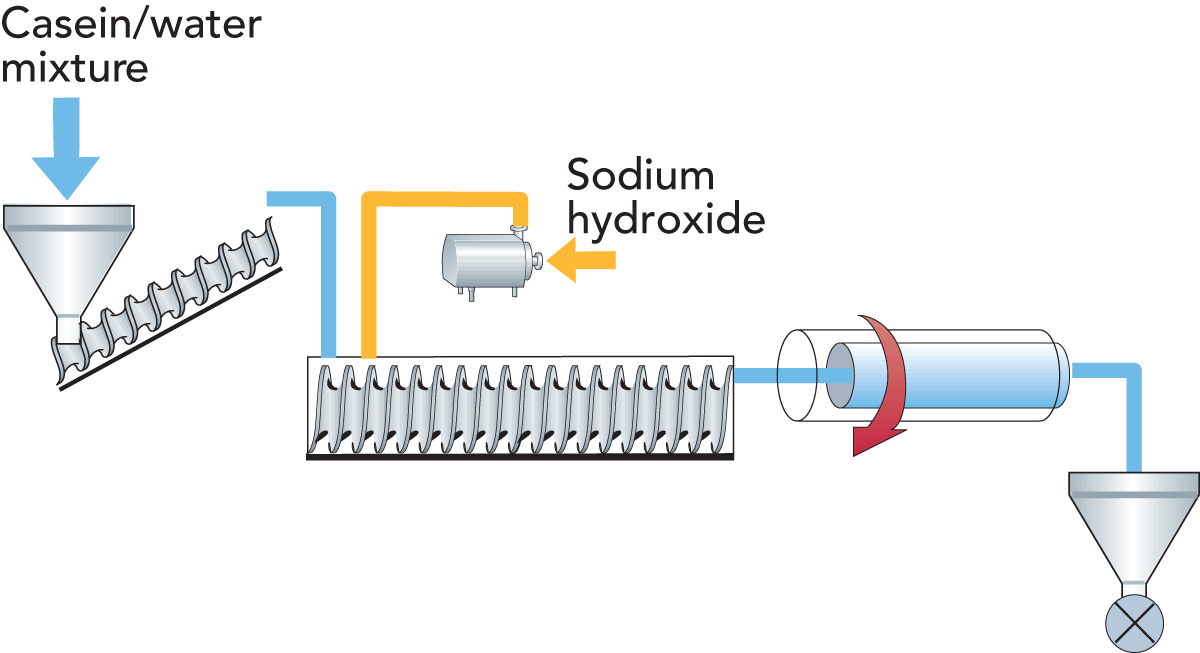
Extruded sodium caseinate
It is possible to produce sodium caseinate from casein in the presence of a limited amount of water by using extrusion techniques.
Some European companies dealing with extrusion cooking – Werner & Pfleiderer GmbH (Germany), Clextral (France) and a few others – report good results from production of sodium caseinate by extrusion cooking.
Most of the published information gives dry casein as the starting material. Water and alkali are added to form a mixture for extrusion. The casein/water mixture may have a moisture content of
10 – 30%.
The extrusion technique used in the production of caseinates is likely to become highly competitive with the traditional batch technique.
Furthermore, extrusion processing has also been tested in production of acid casein from skim milk powder. J Fichtali and F R van der Vort have run trials in a pilot plant at the MacDonald College of McGill University, Quebec, Canada. They summarize the results of their trials (1990) as follows:
"Our initial work on the production of an acid curd from SMP (skim milk powder) by extrusion processing indicated that significantly more effort had to go into developing the process to produce a quality product. The United States, Canada and the European Economic Community have at times experienced a chronic oversupply of milk, of which substantial amounts are converted into skim milk powder. By modifying the extrusion process conditions, studying high solids coagulation and optimizing the coagulation and washing steps, acid casein of an acceptable quality can be produced by extrusion. This process is continuous, controllable, uses high solid SMP and may reduce labour and floor space requirements relative to conventional processes. This material can serve as a feed for further conversion by extrusion to sodium caseinate, which will be discussed in a succeeding paper."
Uses of caseins and caseinates
Rennet casein
Rennet casein is a product different from acid casein. In industry, it is used principally in the production of artificial substances in the plastics category. Casein polymerized with formalin is known as galalith, and synthetic fibres of casein are known as lanital. In spite of the large supply of various plastics which compete directly with galalith, there is still some demand for casein for galalith production. Small quantities of rennet casein are also used as a raw material for processed cheese. Rennet casein is insoluble in water.
Acid casein
Acid casein dominates the world markets. It is used in the chemical industry as an additive in paper manufacture for the glazing of paper of fine quality. For paper industry applications, it is particularly important that the casein is free from fat and contains no particles of foreign or burnt matter that might make spots on the paper. To obtain extremely low fat content in skim milk, it should be passed through a microfiltration plant (MF) in combination with pasteurization. Each industry has its own strict quality specifications. The paint and cosmetic industries are also large users of casein.
Sodium caseinate
A casein application of growing importance is its use as a raw material for the manufacture of sodium caseinate. The casein is easily dissolved in a diluted alkali, and the liquid is then spray-dried to a powder. This powder is much more soluble than casein and is being increasingly used by the food industry. It is often used as an emulsifier in cured meats and is found in a number of new products, such as milk and cream substitutes.
As sodium caseinate is highly viscous when dissolved, the maximum obtainable concentration is 20% at 55 – 60 °C.
Calcium caseinate
For certain applications, calcium caseinate may be chosen instead of sodium caseinate, one reason being the wish to reduce the sodium content of the product to a minimum.
The viscosity of calcium caseinate is somewhat lower than that of sodium caseinate at the same concentration.
Calcium co-precipitate
This product can also be dissolved in alkali and spray dried, and has much the same field of application as caseinate, however, in the production of calcium co-precipitate, it is possible to adapt the process for the purpose of regulating colour, solubility, and ash content in closer conformity to the users’ requirements.
One of the most important advantages of casein and caseinate from a nutritional point of view is the relatively high content of the essential amino acid lysine. Moreover, tests have shown that the lysine keeps much longer, thanks to the absence of lactose in the environment. This suggests that milk proteins can be more conveniently stored in the form of casein and caseinate than, for example, as dried milk powder.
Casein produced for industrial use must satisfy long-established demands for chemical purity. The new trend shows that casein and precipitate are intermediate products which find their way into a host of food products and must therefore satisfy strict demands with respect to bacteriological as well as chemical purity.
Process lines must be designed and constructed so that they ensure hygienic manufacturing conditions. As casein is much more of a seasonal product than many other dairy products, the possibility of running the production line in multiple shifts without an undue demand for manual labour must be provided. Water consumption must also be kept within reasonable limits.
Therefore, in these circumstances, it is of interest to be able to plan continuous production lines, e.g. incorporating centrifugal machines for dewatering the casein and recovery of casein losses from the whey and wash water.
References: A large part of the information concerning caseinates is drawn from a review made by C R Southward,
NZDRI, and published in the N.Z.J. of D. Science and Technology, 20, 79 – 101 (1985).